Abstract
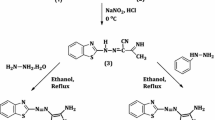
As the raw materials of organic–inorganic dyes, 2-triethoxysilylazulene derivatives (2a–2d) were synthesized via a cross-coupling reaction between a 2-haloazulene derivative (1a–1d) and triethoxysilane in the presence of a rhodium catalyst. Compounds 2a–2d were obtained as colored liquids and characterized using NMR and mass spectroscopy. The UV–Vis spectra of 2a–2d were red-shifted as compared to that of hydrogen-substituted azulene derivatives (3a–3d). This was attributed to the extended conjugated system and specific properties of the azulene moiety. Compound 2a was polymerized via a hydrolysis–condensation reaction, and the resulting polymer (P2a) was characterized using GPC, 29Si NMR, and FT-IR spectroscopy. The UV–Vis spectrum of P2a was red-shifted as compared to that of 2a, which was attributed to the π–π interactions.

2-Triethoxysilylazulene derivatives show excellent chromogenic properties. The absorption wavelength is dependent on the type of functional groups located at the 1,3-position.
Highlights
-
2-Triethoxysilylazulene derivatives were synthesized via a cross-coupling reaction between a 2-haloazulene derivative and triethoxysilane.
-
The UV–Vis spectra of these derivatives are dependent on the type of functional groups.
-
2-Triethoxysilylazulene polymer was prepared via a hydrolysis–condensation reaction.
-
The UV–Vis spectrum of the polymer was slightly red-shifted as compared to that of the monomer.





Similar content being viewed by others
References
Ruiz-Hitzky E, Aranda P, Darder M, Rytwo G (2010) J Mater Chem 20:9306–9321
Sánchez del Río M, Martinetto P, Reyes-Valerio C, Doopyhée E, Suárez M (2006) Archaeometry 48:115–130
Sanchez C, Boissiere C, Cassaignon S, Chaneac C, Durupthy O, Faustini M, Grosso D, Laberty-Robert C, Nicole L, Portehault D, Ribot F, Rozes L, Sassoye C (2014) Chem Mater 26:221–238
Choi DH, Park JH, Lee JH, Lee SD (2000) Thin Solid Films 360:213–221
Chaput F, Riehl D, Boilot JP, Cargnelli K, Canva M, Lévy Y, Brun A (1996) Chem Mater 8:312–314
Serwadczak M, Kucharski S (2006) J Sol–Gel Sci Technol 37:57–62
Demirel GB, Dilsiz N, Çakmak M, Çaykara T (2011) J Mater Chem 21:3189–3196
McDonald RN, Richmond JM, Curtis JR, Petty HE, Hoskins TL (1976) J Org Chem 41:1811–1821
Anderson Jr. AG, Steckler BM (1959) J Am Chem Soc 81:4941–4946
Lemal D, Goldman G (1988) J Chem Educ 65:923–925
Michl J, Thulstrup E (1976) Tetrahedron 32:205–209
Yamaguchi Y, Ogawa K, Nakayama K, Ohba Y, Katagiri H (2013) J Am Chem Soc 135:19095–19098
Becke AD (1988) Phys Rev A 38:3098–3100
Becke AD (1993) J Chem Phys 98:5648–5652
Perdew JP, Wang Y (1992) Phys Rev B 45:13244–13249
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford, CT
Armarego WLF, Chai C (2012) Purification of laboratory chemicals, 7th ed. Butterworth-Heinemann, Oxford, UK.
Zhang J, Petoud S (2008) Chem Eur J 14:1264–1272
Koch M, Blacque O, Venkatesan K (2012) Org Lett 14:1580–1583
Ito S, Nomura A, Morita N, Kabuto C, Kobayashi H, Maejima S, Fujimori K, Yasunami M (2002) J Org Chem 67:7295–7302
Nozoe T, Imafuku K, Yin B, Honda M, Goto Y, Hara Y, Andoh T, Yamamoto H (1988) Bull Chem Soc Jpn 61:2531–2539
Nozoe T, Takase K, Kato M, Nogi T (1971) Tetrahedron 27:6023–6035
Nihon University (2008) Process for production of azulene derivatives and azulene derivatives. Japan patent JP 2008-285435A.
Bing LB, Yun-Shan L (2010) J Heterocycl Chem 48:205–208
McDonald RN, Richmond JM, Curtis JR, Petty HE, Hoskins TL (1976) J Org Chem 41:1811–1821
Morita T, Takase K (1982) Bull Chem Soc Jpn 55:1144–1152
Ueno T, Toda H, Yasunami M, Yoshifuji M (1996) Bull Chem Soc Jpn 69:1645–1656
Murata M, Yamasaki H, Ueta T, Nagata M, Ishikura M, Watanabe S, Masuda Y (2007) Tetrahedron 63:4087–4094
Murata M, Ishikura M, Nagata M, Watanabe S, Masuda Y (2002) Org Lett 4:1843–1845
Yamanoi Y, Nishihara H (2006) Tetrahedron Lett 47:7157–7161
Seganish WM, Handy CJ, DeShong P (2005) J Org Chem 70:8948–8955
Manoso AS, DeShong P (2001) J Org Chem 66:7449–7455
Denmark SE, Kallemeyn JM (2003) Org Lett 5:3483–3486
Handy CJ, Manoso AS, McElroy WT, Seganish WM, DeShong P (2005) Tetrahedron 61:12201–12225
Tétreault N, Muthyala RS, Liu RSH, Steer RP (1999) J Phys Chem A 103:2524–2531
Shevyakov SV, Li H, Muthyala R, Asato AE, Croney JC, Jameson DM, Liu RSH (2003) J Phys Chem A 107:3295–3299
Patalinghug WC, Chang M, Solis J (2007) J Chem Educ 84:1945–1947
Foggi P, Neuwahl FVR, Moroni L, Salvi PR (2003) J Phys Chem A 107:1689–1696
Walton DRM (1965) J Organomet Chem 3:438–441
Maeda H, Maeda T, Mizuno K (2012) Molecules 17:5108–5125
Abe Y, Gunji T (2004) Prog Polym Sci 29:149–182
Gunji T, Tozune T, Kaburaki H, Arimitsu K, Abe Y (2013) J Polym Sci A 51:4732–4741
Gunji T, Kaburagi H, Tsukada S, Abe Y (2015) J Sol–Gel Sci Technol 75:564–573
Hayami R, Nishikawa I, Hisa T, Nakashima H, Sato Y, Ideno Y, Sagawa T, Tsukada S, Yamamoto K, Gunji T (2018) J Sol–Gel Sci Technol 88:660–670
Yoldas BE (1986) J Non-Cryst Solids 82:11–23
Sun X, Xu Y, Jiang D, Yang D, Wu D, Sun Y, Yang Y, Yuan H, Deng F (2006) Colloids Surf A 289:149–157
Yoshinaga I, Yamada N, Katayama S (2003) J Sol–Gel Sci Technol 28:65–70
Kuniyoshi M, Takahashi M, Tokuda Y, Yoko T (2006) J Sol–Gel Sci Technol 39:175–183
Olejniczak Z, Łęczka M, Cholewa-Kowalska K, Wojtach K, Rokita M, Mozgawa W (2005) J Mol Struct 744–747:465–471
Mori H, Yamada M (2012) Colloid Polym Sci 290:1879–1891
Pescarmona PP, Maschmeyer T (2001) Aust J Chem 54:583–596
Li YS, Wang Y, Ceesay S (2009) Spectrochim Acta A 71:1819–1824
Amicangelo JC, Leenstra WR (2003) J Am Chem Soc 125:14698–14699
Murai M, Amir E, Amir RJ, Hawker CJ (2012) Chem Sci 3:2721–2725
Zhuo D, Gu A, Liang G, Hu JT, Zhou C, Yuan L (2011) Polym Adv Technol 22:2617–2625
Chang CC, Huang FH, Lin ZM, Cheng LF (2015) J Coat Technol Res 12:731–738
Seifert A, Ladewig K, Schönherr P, Hofmann K, Lungwitz R, Roth I, Pohlers A, Hoyer W, Baumann G, Schulze S, Hietschold M, Moszner N, Burtscher P, Spange S (2010) J Sol–Gel Sci Technol 53:328–341
Jain S, Goossens JGP, van Duin M (2006) Macromol Symp 233:225–234
Park ES, Ro HW, Nguyen CV, Jaffe RL, Yoon DY (2008) Chem Mater 20:1548–1554
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hayami, R., Izumiya, T., Kokaji, T. et al. 2-Triethoxysilylazulene derivatives: Syntheses and optical properties, and hydrolysis—condensation of 2-triethoxysilylazulene. J Sol-Gel Sci Technol 91, 399–406 (2019). https://doi.org/10.1007/s10971-019-04991-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04991-8