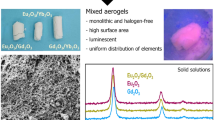
Abstract
Synthesis of chlorine-free, rare earth oxide aerogels from the lanthanide series was achieved using a modified epoxide-assisted sol-gel method. An ethanolic solution of the hydrated metal nitrate, propylene oxide, and ammonium carbonate was found to gel upon heating to 333 K. Critical point drying of the wet gel in CO2 yielded monolithic aerogels. Most of the aerogels were amorphous as-prepared, but became nano-crystalline after calcination at 923 K in air. The aerogels had high surface areas (up to 150 m2/g), low densities (40–225 mg/cm3), and were photoluminescent.

Highlights
-
Rare earth oxide aerogels were prepared by epoxide-assisted sol-gel route.
-
Rare earth oxide aerogels are monolithic, chlorine-free, and possess large surface areas.
-
Calcination at 923 K results in nano-crystalline aerogels, with particles less than 25 nm in diameter.
-
Characterization of these aerogels includes photoluminescence spectroscopy, Rietveld refinements, and electron microscopy.










Similar content being viewed by others
References
McFarland EW, Metiu H (2013) Catalysis by doped oxides. Chem Rev 113:4391
Ranjit KT, Cohen H, Willner I, Bossmann S, Braun AM (1999) Lanthanide oxide-doped titanium dioxide: effective photocatalysts for the degradation of organic pollutants. J Mater Sci 34:5273
Sauer J, Marlow F, Spliethoff B, Schüth F (2002) Rare earth oxide coating of the walls of SBA-15. Chem Mater 14:217
Skårman B, Grandjean D, Benfield RE, Hinz A, Andersson A, Wallenberg LR (2002) Carbon monoxide oxidation on nanostructured CuOx/CeO2 composite particles characterized by HREM, XPS, XAS, and high-energy diffraction. J Catal 211:119
Van der Avert P, Weckhuysen BM (2002) Low-temperature destruction of chlorinated hydrocarbons over lanthanide oxide based catalysts Angew Chem Int Ed 41:4730
Borchert Y, Sonström P, Wilhelm M, Borchert H, Bäumer M (2008) Nanostructured praseodymium oxide: preparation, structure, and catalytic properties. J Phys Chem C 112:3054
Reddy BM, Thrimurthulu G, Katta L, Yamada Y, Park S-E (2009) Structural characteristics and catalytic activity of nanocrystalline ceria–praseodymia solid solutions. J Phys Chem C 113:15882
Zhang HD, Li B, Zheng QX, Jiang MH, Tao XT (2008) Synthesis and characterization of monolithic Gd2O3 aerogels. J Non-Cryst Solids 354:4089
Zhang Y, Lei Z, Li J, Lu S (2001) A new route to three-dimensionally well-ordered macroporous rare-earth oxides. New J Chem 25:1118
Tillotson TM, Sunderland WE, Thomas IM, Hrubesh LW (1994) Synthesis of lanthanide and lanthanide-silicate aerogels. J Sol-Gel Sci Technol 1:241
Clapsaddle BJ, Neumann B, Wittstock A, Sprehn DW, Gash A, Satcher JH, Simpson RL, B„umer M (2012) A sol-gel methodology for the preparation of lanthanide-oxide aerogels: preparation and characterization. J Sol-Gel Sci Technol 64:381
Hutchings GJ, King F, Okoye IP, Rochester CH (1994) Influence of chlorine poisoning of copper/alumina catalyst on the selective hydrogenation of crotonaldehyde. Catal Lett 23:127
Heldal JA, Mørk PC (1982) Chlorine-containing compounds as copper catalyst poisons. J Am Oil Chem Soc 59:396
Shin E-J, Spiller A, Tavoularis G, Keane MA (1999) Chlorine–nickel interactions in gas phase catalytic hydrodechlorination: catalyst deactivation and the nature of reactive hydrogen. Phys Chem Chem Phys 1:3173
Gregg SJ, Sing KSW (1982) Adsorption, surface area, and porosity. 2nd edn. Academic, London
Gash AE, Tillotson TM, Satcher JH, Poco JF, Hrubesh LW, Simpson RL (2001) Use of epoxides in the sol-gel synthesis of porous iron(III) oxide monoliths from Fe(III) salts. Chem Mater 13:999
Gash AE, Satcher JH, Simpson RL (2003) Strong akaganeite aerogel monoliths using epoxides: synthesis and characterization. Chem Mater 15:3268
Baumann TF, Gash AE, Chinn SC, Sawvel AM, Maxwell RS, Satcher JH (2005) Synthesis of high-surface-area alumina aerogels without the use of alkoxide precursors. Chem Mater 17:395
Poco JF, Satcher JH, Hrubesh LW (2001) Synthesis of high porosity, monolithic alumina aerogels. J Non-Cryst Solids 285:57
Teck M, Murshed MM, Schowalter M, Lefeld N, Grossmann HK, Grieb T, Hartmann T, Robben L, Rosenauer A, Mädler L, Gesing TM (2017) Structural and spectroscopic comparison between polycrystalline, nanocrystalline and quantum dot visible light photo-catalyst Bi2WO6. J Solid State Chem 254:82
Aldebert P, Traverse JP (1979) Etude par diffraction neutronique des structures de haute temperature de La2O3 et Nd2O3. Mater Res Bull 14:303
Andreeva AF, Gil’man IY, Gamarnik MY, Dekhtyaruk VI (1986) Structure and some optical properties of films of Pr6O11. Inorg Mater 22:1155
Antic B, Mitric M, Rodic D (1995) Structure properties and magnetic susceptibility of diluted magnetic semiconductor Y2−xHoxO3. J Magn Magn Mater 145:349
Antic B, Oennerud P, Rodic D, Tellgren R (1993) The structure characteristics of the diluted magnetic semiconductor Y2−xDyxO3. Powder Diffr 8:216
Artini C, Pani M, Plaisier JR, Costa GA (2014) Structural study of Nd oxidation by means of in-situ synchrotron X-ray diffraction. Solid State Ion 257:38
Atou T, Kusaba K, Fukuoka K, Kikuchi M, Syono Y (1990) Shock-induced phase transition of M2O3-type compounds. J Solid State Chem 89:378
Atou T, Kusaba K, Tsuchida Y, Utsumi W, Yagi T, Syono Y (1989) Reversible B-type–A-type transition of Sm2O3 under high pressure. Mater Res Bull 24:1171
Bartos A, Lieb KP, Uhrmacher M, Wiarda D (1993) Refinement of atomic positions in bixbyite oxides using perturbed angular correlation spectroscopy. Acta Crystallogr B 49:165
Ben Farhat L, Amami M, Hlil EK, Ben Hassen R (2009) Structural and vibrational study of C-type doped rare earth sesquioxide. J Alloy Compd 479:594
Ben Farhat L, Amami M, Hlil EK, Ben Hassen R (2010) Synthesis, structure and magnetic properties of the mixed system. Mater Chem Phys 123:737
Bevan DJM (1955) Ordered intermediate phases in the system CeO2–Ce2O3. J Inorg Nucl Chem 1:49
Bischof R, Kaldis E, Lacis I (1983) Crystal growth os ytterbium dihydride and the phase relations in the Yb–H system. J Less-Common Met 94:117
Blanusa J, Mitric M, Felner I, Jovic N, Bradaric I (2003) The crystal structure refinement and magnetic susceptibility of La2−xErxO3. J Magn Magn Mater 263:295
Blanusa J, Mitric M, Rodic D, Szytula A, Slaski M (2000) An X-ray diffraction and magnetic susceptibility study of TmxY2−xO3. J Magn Magn Mater 213:75
Bommer H (1939) Die Gitterkonstanten der C-Formen der Oxyde der seltenen Erdmetalle. Z Anorg Allg Chem 241:273
Boucherle JX, Schweizer J (1975) Refinement of the Nd2O3 structure and determination of the neutron scattering length of neodymium. Acta Crystallogr B 31:2745
Boulesteix C, Pardo B, Caro PE, Gasgnier M, la Blanchetais CH (1971) Etude de couches minces de sesquioxyde de samarium type par microscopie et diffraction electroniques. Acta Crystallogr B 27:216
Chandrasekhar M, Nagabhushana H, Sudheerkumar KH, Dhananjaya N, Sharma SC, Kavyashree D, Shivakumara C, Nagabhushana BM (2014) Comparison of structural and luminescence properties of Dy2O3 nanopowders synthesized by co-precipitation and green combustion routes. Mater Res Bull 55:237
Chandrasekhar M, Sunitha DV, Dhananjaya N, Nagabhushana H, Sharma SC, Nagabushana BM, Shivakumara C, Chakradhar RPS (2012) Structural and phase dependent thermo and photoluminiscent properties of Dy3 and DyO3 nanorods. Mater Res Bull 47:2085
Chen G, Peterson JR, Brister KE (1994) An energy-dispersive X-ray diffraction study of monoclinic Eu2O3 under pressure. J Solid State Chem 111:437
Chikalla TD, McNeilly CE, Roberts FP (1972) Polymorphic modifications of Pm2O3. J Am Ceram Soc 55:428
Cromer DT (1957) The crystal structure of monoclinic Sm2O3. J Phys Chem 61:753
Cunningham GW (1963) Nuclear poisons. React Mater 6:63
Dordevic V, Antic Z, Nikolic MG, Dramicanin MD (2014) Comparative structural and photoluminescent study of Eu-doped La2O3 and La3 nanocrystalline powders. J Phys Chem Solids 75:276
Faucher M, Pannetier J, Charreire Y, Caro P (1982) Refinement of the Nd2O3 and Nd2O2S structures at 4 K. Acta Crystallogr B 38:344
Felsche J (1969) A new form of La2O3. Naturwissenschaften 56:212
Ferguson IF (1975) Lattice parameters of oxides and mixed oxides with the monoclinic rare earth type B structure. Acta Crystallogr A 31:S69
Fert A (1962) Structure de quelques oxydes de terres rares. Bull Fr Soc Mineral Cristallogr 85:267
Gasgnier M, Schiffmacher G, Caro P, Eyring L (1986) The formation of rare earth oxides far from equilibrium. J Less-Common Met 116:31
Gouteron J, Michel D, Lejus AM, Zarembowitch J (1981) Raman spectra of lanthanide sesquioxide single crystals: correlation between A and B-type structures. J Solid State Chem 38:288
Greis O, Ziel R, Breidenstein B, Haase A, Petzel T (1994) The crystal structure of the low-temperature A-type modification of Pr2O3 from X-ray powder and electron single crystal diffraction. J Alloy Compd 216:255
Gupta ML, Singh S (1970) Thermal expansion of CeO2, Ho2O3, and Lu2O3 from 100 Å to 300 Å K by an X-ray method. J Am Ceram Soc 53:663
Guzik M, Pejchal J, Yoshikawa A, Ito A, Goto T, Siczek M, Lis T, Boulon G (2014) Structural investigations of Lu2O3 as single crystal and polycrystalline transparent ceramic. Cryst Growth Des 14:3327
Hase W (1963) Neutronographische Bestimmung der Kristallstrukturparameter von Dy2O3, Tm2O3 und alpha-Mn2O3. Phys Status Solidi 3:446
Heiba Z, Okuyucu H, Hascicek YS (2002) X-ray structure determination of the rare earth oxides (Er1−uGdu)2O3 applying the Rietveld method. J Appl Crystallogr 35:577
Heiba ZK, Akin Y, Sigmund W, Hascicek YS (2003) X-ray structure and microstructure determination of the mixed sesquioxides (Eu1−xYbx)2O3 prepared by a sol-gel process. J Appl Crystallogr 36:1411
Heiba ZK, Arda L (2008) X-ray diffraction analysis of powder and thin film of (Gd1−xYx)2O3 prepared by sol-gel process. Cryst Res Technol 43:282
Heiba ZK, Arda L, Hascicek YS (2005) Structure and microstructure characterization of the mixed sesquioxides (Gd1−xYbx)2O3 and (Gd1−xHox)2O3 prepared by sol-gel process. J Appl Crystallogr 38:306
Heiba ZK, Bakr Mohamed M, Fuess H (2012) XRD, IR and Raman investigations of structural properties of Dy2−xHoxO3 prepared by sol gel procedure. Cryst Res Technol 47:535
Hering SA, Huppertz H (2009) High-pressure synthesis and crystal structures of monoclinic B-Ho2O3 and orthorhombic HoGaO3. Z Naturforsch B: Chem Sci 64:1032
Hubbert-Paletta E, Müller-Buschbaum H (1968) Roentgenographische Untersuchung an Einkristallen von monoklinem Tb2O3. Z Anorg Allg Chem 363:145
Ishibashi H, Shimomoto K, Nakahigashi K (1994) Electron density distribution and chemical bonding of Ln2O3 from powder x-ray diffraction data by the maximum-entropy method. J Phys Chem Solids 55:809
Kashaev AA, Ushchapovskii LV, Il’in AG (1975) Electron diffraction and X-ray diffraction study of rare earth metal oxides in thin films. Kristallografiya 20:192
Katari V, Achary SN, Deshpande SK, Babu PD, Sinha AK, Salunke HG, Gupta N, Tyagi AK (2014) Effect of annealing environment on low-temperature magnetic and dielectric properties of EuCo0.5Mn0.5O3. J Phys Chem C 118:17900
Kennedy BJ, Avdeev M (2011) The structure of C-type Gd2O3. A powder neutron diffraction study using enriched Gd Aust J Chem 64:119
Kennedy BJ, Avdeev M (2011) The structure of B-type Sm2O3. A powder neutron diffraction study using enriched 154Sm. Solid State Sci 13:1701
Koehler WC, Wollan EO (1953) Neutron-diffraction study of the structure of the A-form of the rare earth sesquioxides. Acta Crystallogr 6:741
Koehler WC, Wollan EO, Wilkinson MK (1958) Paramagnetic and nuclear scattering cross sections of holmium sesquioxyde. Phys Rev 110:37
Kohlmann H, Hein C, Kautenburger R, Hansen TC, Ritter C, Doyle S (2016) Crystal structure of monoclinic samarium and cubic europium sesquioxides and bound coherent neutron scattering lengths of the isotopes 154Sm and 153Eu. Z Kristal Cryst Mater 231:517
Whiffen RK, Antic Z, Speghini A, Brik MG, Bartova B, Bettinelli M, Dramicanin MD (2014) Structural and spectroscopic studies of Eu doped Lu2O3–Gd2O3 solid solutions. Opt Mater 36:1083
Lejus AM, Bernier JC, Collongues R (1976) Elaboration et proprietes magnetiques de monocristaux d’oxyde de praseodyme Pr2O3. J Solid State Chem 16:349
Malinovskii YA, Bondareva OS (1991) Refined crystal structure of Er2O3. Kristallografiya 36:1558
Maslen EN, Strel’tsov VA, Ishizawa N (1996) A synchrotron X-ray study of the electron density in C-type rare earth oxides. Acta Crystallogr B 52:414
McCarthy GJ (1971) Approximations in refinement of elastic constant values from thermal diffuse scattering measurements. J Appl Crystallogr 4:399
Mitric M, Blanusa J, Barudzija T, Jaglicic Z, Kusigerski V, Spasojevic V (2009) Magnetic properties of trivalent Sm ions in SmxY2−xO3. J Alloy Compd 485:473
Moon RM, Koehler WC, Child HR, Raubenheimer LJ (1968) Magnetic structures of Er2O3 and Yb2O3. Phys Rev 176:722
Müller-Buschbaum H (1966) Zur Struktur der A-Form der Sesquioxide der Seltenen Erden. II Strukturuntersuchung an Nd2O3. Z Anorg Allg Chem 343:6
Müller-Buschbaum H, von Schnering HG (1965) Strukturuntersuchungen an La2O3. Z Anorg Allg Chem 340:232
Pauling L (1928) The crystal structure of the A-modification of the rare earths sesquioxides. Z Kristallogr Kristallogeom Kristallophys Kristallochem 69:415
Pedroso CCS, Carvalho JM, Rodrigues LCV, Holsa J, Brito HF (2016) Rapid and energy-saving microwave-assisted solid-state synthesis of Pr3+-, Eu3+-, or Tb3+-doped Lu2O3 persistent luminescence materials. ACS Appl Mater Interfaces 8:19593
Pires AM, Davolos MR, Paiva-Santos CO, Berwerth Stucchi E, Flor J (2003) New X-ray powder diffraction data and Rietveld refinement for Gd2O3 monodispersed fine spherical particles. J Solid State Chem 171:420
Post B, Moskowitz D, Glaser FW (1956) Borides of rare earth and related metals. Planseeber Pulvermetall 1955:173
Rudenko VS, Boganov AG (1970) Reduction cycle MO2–M2O3 for cerium, praseodymium, and terbium oxides. Inorg Mater 6:1893
Rudenko VS, Boganov AG (1970) Stoichiometry and phase transitions in rare earth oxides. Inorg Mater 6:1893
Rudenko VS, Boganov AG (1973) The observation of face centred cubic Gd, Tb, Dy, Ho, Er and Tm in the form of thin films and their oxidation. J Phys F 3:1
Saiki A, Ishizawa N, Mizutani N, Kato M (1985) Structural change of C-rare Earth sesquioxides Yb2O3 and Er2O3 as a function of temperature. Yogyo Kyokai Shi 93:649
Scavini M, Coduri M, Allieta M, Brunelli M, Ferrero C (2012) Probing complex disorder in Ce1−xGdxO2−x/2 using the pair distribution function analysis. Chem Mater 24:1338
Schiller G (1985) Die Kristallstrukturen von Ce2O3, LiCeO2 und CeF3—Ein Beitrag zur Kristallchemie des dreiwertigen Cers. Dissertation Universitaet Karlsruhe 1985:1
Schleid T, Meyer G (1989) Single crystals of rare earth oxides from reducing halide melts. J Less-Common Met 149:73
Sheu HS, Shih WJ, Chuang WT, Li IF, Yeh CS (2010) Crystal structure and phase transition of GdOH studied by synchrotron powder diffraction. J Chin Chem Soc 57:938
Singh HP, Dayal B (1969) Precise determination of the lattice parameters of holmium and erbium sesquioxides at elevated temperatures. J Less-Common Met 18:172
Taylor D (1984) Thermal expansion data: III Sesquioxides, M2O3, with the corundum and the A-, B- and C - M2O3 structures. Trans J Br Ceram Soc 83:92
Turcotte RP, Warmkessel JM, Tilley RJD, Eyring L (1971) On the phase interval PrO1.50 to PrO1.71 in the praseodymium oxide–oxygen system. J Solid State Chem 3:265
Umesh B, Eraiah B, Nagabhushana H, Nagabhushana BM, Nagaraja G, Shivakumara C, Chakradhar RPS (2011) Synthesis and characterization of spherical and rod like nanocrystalline Nd2O3 phosphors. J Alloy Compd 509:11436
Vasundhara K, Achary SN, Patwe SJ, Sahu AK, Manoj N, Tyagi AK (2014) Structural and oxide ion conductivity studies on Yb1−xBixO1.5 composites. J Alloy Compd 596:151
Vucinic-Vasic M, Kremenovic A, Nikolic AS, Colomban P, Mazzerolles L, Kahlenberg V, Antic B (2010) Core and shell structure of ytterbium sesquioxide nanoparticles. J Alloy Compd 502:107
Will G, Masciocchi N, Hart M, Parrish W (1987) Ytterbium L-edge anomalous scattering measured with synchrotron radiation powder diffraction. Acta Crystallogr Sect A: Found Crystallogr 43:677
Wolf R, Hoppe R (1985) Eine Notiz zum A-Typ der Lanthanoidoxide: Ueber Pr2O3. Z Anorg Allg Chem 529:61
Wontcheu J, Schleid T (2008) Single crystals of B-type Er2O3. Z Anorg Allg Chem 634:2091
Yakel HL (1979) A refinement of the crystal structure of monoclinic europium sesquioxide. Acta Crystallogr B 35:564
Zachariasen WH (1926) Die Kristallstruktur der alpha-Modifikation von den Sesquioxiden der seltenen Erdmetalle. Z Phys Chem 123:134
Zachariasen WH (1927) The crystal structure of the modification C of the sesquioxides of the rare earth metals, and of indium and thallium. Nor Geol Tidsskr 9:310
Zachariasen WH (1928) Untersuchungen ueber die Kristallstruktur von Sesquioxyden und Verbindungen ABO3. Skrifter utgitt av det Norske Videnskaps-Akademi i Oslo. 1, Matematisk-Naturvidenskapelig Klasse 1928:1
Zav’yalova AA, Imamov RM, Ragimli NA, Semiletov SA (1976) Electron-diffraction study of the structure of cubic C-Sm2O3. Kristallografiya 21:727
Zhang FX, Lang M, Wang JW, Becker U, Ewing RC (2008) Structural phase transition of cubic Gd2O3 at high pressures. Phys Rev Ser 3 B Condens Matter 78:064114
Molina C, Ferreira RAS, Poirier G, Fu L, Ribeiro SJL, Messsaddeq Y, Carlos LD (2008) Er3+-based diureasil organic–inorganic hybrids. J Phys Chem C 112:19346
Planelles-Arago J, Cordoncillo E, Ferreira RAS, Carlos LD, Escribano P (2011) Synthesis, characterization and optical studies on lanthanide-doped CdS quantum dots: new insights on CdS [rightward arrow] lanthanide energy transfer mechanisms. J Mater Chem 21:1162
Miguel A, Azkargorta J, Morea R, Iparraguirre I, Gonzalo J, Fernandez J, Balda R (2013) Spectral study of the stimulated emission of Nd3+ in fluorotellurite bulk glass. Opt Express 21:9298
Acknowledgements
We like to thank the German Science Foundation (DFG) for financial support in the scientific large instrument program under the project number INST144/435-1FUGG. JI and MB gratefully acknowledge funding by the DFG through the graduate school 1860 “Micro-, meso- and macroporous nonmetallic Materials: Fundamentals and Applications”. This work is partially developed in the scope of the projects CICECO—Aveiro Institute of Materials (UID/CTM/50011/2013) financed by national funds through the Fundação para a Ciência e a Tecnologia/Ministério da Educação e Ciência (FCT/MEC) and when applicable co-financed by FEDER under the PT2020 Partnership Agreement. We acknowledge the Fraunhofer Institute for Manufacturing Technology and Advanced Materials (Bremen, Germany) for the provision of access to their TEM facility and Karsten Thiel for assistance. This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344, through LDRD awards 13-LW-099 and 16-ERD-051.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Worsley, M.A., Ilsemann, J., Gesing, T.M. et al. Chlorine-free, monolithic lanthanide series rare earth oxide aerogels via epoxide-assisted sol-gel method. J Sol-Gel Sci Technol 89, 176–188 (2019). https://doi.org/10.1007/s10971-018-4811-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4811-y