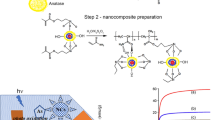
Abstract
In this study, we prepared an adsorbent layer for adsorption heat pump by using ferroaluminophosphate (FAPO) particles and inorganic silica binder and characterized the physical properties thereof. We tried two ideas in preparing the adsorbent layer: dual precursor for binder and surface modification of the FAPO particles. The inorganic silica binder was prepared through a sol–gel process by using tetraethoxysilane (TEOS) and methyltriethoxysilane (MTES) as the dual precursor. The surface modification by TEOS molecules on the FAPO particles was done to promote the reactivity between particles and binder. The surface modification to form monolayer of silane was carefully done in a hydrophobic solvent, cyclohexane, preventing the supply of water molecules into the solution. As a result, both the use of dual precursor binder and the surface modification effectively improved the mechanical stability of the layer as shown by higher resistance to mechanical vibration. It was analyzed that the addition of MTES served to enhance the mechanical stability of the adsorbent layer by providing the flexible element, Si–C bonding, in the rigid inorganic matrix. To explain the microstructure analysis by SEM and TEM, and the zeta potential measurement, we proposed a surface reaction model comprising the formation of very thin layer of surface silanol on FAPO particles enhancing the reactivity between the particle and the binder. Both the dual precursor and the surface modification produced no noticeable detrimental effect on the key properties such as water vapor adsorption and thermal conductivity.
Graphical Abstract










Similar content being viewed by others
References
Demir H, Mobedi M, Ülkü S (2008) A review on adsorption heat pump: problems and solutions. Renew Sustain Energy Rev 12:2381–2403. doi:10.1016/j.rser.2007.06.005
Wu W, Wang B, Shi W, Li X (2014) Absorption heating technologies: a review and perspective. Appl Energy 130:51–71. doi:10.1016/j.apenergy.2014.05.027
Aristov YI (2013) Challenging offers of material science for adsorption heat transformation: a review. Appl Therm Eng 50:1610–1618. doi:10.1016/j.applthermaleng.2011.09.003
Anyanwu EE (2003) Review of solid adsorption solar refrigerator I: an overview of the refrigeration cycle. Energy Convers Manag 44:301–312. doi:10.1016/S0196-8904(02)00038-9
Wang SG, Wang RZ, Li XR (2005) Research and development of consolidated adsorbent for adsorption systems. Renew Energy 30:1425–1441. doi:10.1016/j.renene.2004.10.012
Chan CW, Ling-Chin J, Roskilly AP (2013) A review of chemical heat pumps, thermodynamic cycles and thermal energy storage technologies for low grade heat utilisation. Appl Therm Eng 50:1257–1273. doi:10.1016/j.applthermaleng.2012.06.041
Saini VK, Pinto ML, Pires J (2011) Characterization of hierarchical porosity in novel composite monoliths with adsorption studies. Colloids Surf A Physicochem Eng Asp 373:158–166. doi:10.1016/j.colsurfa.2010.10.047
Freni A, Frazzica A, Dawoud B et al (2013) Adsorbent coatings for heat pumping applications: verification of hydrothermal and mechanical stabilities. Appl Therm Eng 50:1658–1663. doi:10.1016/j.applthermaleng.2011.07.010
Bonaccorsi L, Calabrese L, Freni A et al (2013) Zeolites direct synthesis on heat exchangers for adsorption heat pumps. Appl Therm Eng 50:1590–1595. doi:10.1016/j.applthermaleng.2011.10.028
Vasta S, Giacoppo G, Barbera O et al (2014) Innovative zeolite coatings on graphite plates for advanced adsorbers. Appl Therm Eng 72:153–159. doi:10.1016/j.applthermaleng.2014.04.079
Negishi H, Miyamoto A, Endo A (2013) Preparation of thick mesoporous silica coating by electrophoretic deposition with binder addition and its water vapor adsorption–desorption properties. Microporous Mesoporous Mater 180:250–256. doi:10.1016/j.micromeso.2013.06.040
Freni A, Russo F, Vasta S et al (2007) An advanced solid sorption chiller using SWS-1L. Appl Therm Eng 27:2200–2204. doi:10.1016/j.applthermaleng.2005.07.023
Dawoud B (2013) Water vapor adsorption kinetics on small and full scale zeolite coated adsorbers: a comparison. Appl Therm Eng 50:1645–1651. doi:10.1016/j.applthermaleng.2011.07.013
Atakan A, Fueldner G, Munz G et al (2013) Adsorption kinetics and isotherms of zeolite coatings directly crystallized on fibrous plates for heat pump applications. Appl Therm Eng 58:273–280. doi:10.1016/j.applthermaleng.2013.04.037
Sterte J, Mintova S, Zhang G, Schoeman BJ (1997) Thin molecular sieve films on noble metal substrates. Zeolites 18:387–390. doi:10.1016/S0144-2449(97)00032-8
Bonaccorsi L, Bruzzaniti P, Calabrese L et al (2013) Synthesis of SAPO-34 on graphite foams for adsorber heat exchangers. Appl Therm Eng 61:848–852. doi:10.1016/j.applthermaleng.2013.04.053
Freni A, Bonaccorsi L, Calabrese L et al (2015) SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl Therm Eng 82:1–7. doi:10.1016/j.applthermaleng.2015.02.052
Azizi T, Touihri AE, Ben Karoui M, Gharbi R (2016) Comparative study between dye-synthesized solar cells prepared by electrophoretic and doctor blade techniques. Optik (Stuttg) 127:4400–4404. doi:10.1016/j.ijleo.2016.01.191
Lin RY, Chen BS, Chen GL et al (2009) Preparation of porous PMMA/Na+-montmorillonite cation-exchange membranes for cationic dye adsorption. J Membr Sci 326:117–129. doi:10.1016/j.memsci.2008.09.038
Kummer H, Füldner G, Henninger SK (2015) Versatile siloxane based adsorbent coatings for fast water adsorption processes in thermally driven chillers and heat pumps. Appl Therm Eng 85:1–8. doi:10.1016/j.applthermaleng.2015.03.042
Frazzica A, Füldner G, Sapienza A et al (2014) Experimental and theoretical analysis of the kinetic performance of an adsorbent coating composition for use in adsorption chillers and heat pumps. Appl Therm Eng 73:1022–1031. doi:10.1016/j.applthermaleng.2014.09.004
Li A, Thu K, Bin IsmailA et al (2016) Performance of adsorbent-embedded heat exchangers using binder-coating method. Int J Heat Mass Transf 92:149–157. doi:10.1016/j.ijheatmasstransfer.2015.08.097
Nadargi DY, Latthe SS, Hirashima H, Rao AV (2009) Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels. Microporous Mesoporous Mater 117:617–626. doi:10.1016/j.micromeso.2008.08.025
Kim H, Hwang T (2012) Corrosion protection enhancement effect by mixed silica nanoparticles of different sizes incorporated in a sol–gel silica film. J Sol-Gel Sci Technol 63:563–568. doi:10.1007/s10971-012-2820-9
Brinker CJ, Hurd AJ, Schunk PR et al (1992) Review of sol–gel thin film formation. J Non Cryst Solids 147–148:424–436. doi:10.1016/S0022-3093(05)80653-2
Zheng X, Wang RZ, Ge TS, Hu LM (2015) Performance study of SAPO-34 and FAPO-34 desiccants for desiccant coated heat exchanger systems. Energy 93:88–94. doi:10.1016/j.energy.2015.09.024
Parks GA (1965) The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem Rev 65:177–198. doi:10.1021/cr60234a002
Kosmulski M (2001) Chemical properties of material surfaces. Marcel Dekker Inc., New York. doi:10.1201/9780585418049
Kittaka S, Morimoto T (1980) Isoelectric point of metal oxides and binary metal oxides having spinel structure. J Colloid Interface Sci 75:398–403. doi:10.1016/0021-9797(80)90464-6
Kuzniatsova T, Kim Y, Shqau K et al (2007) Zeta potential measurements of zeolite Y: application in homogeneous deposition of particle coatings. Microporous Mesoporous Mater 103:102–107. doi:10.1016/j.micromeso.2007.01.042
Miura T, Miyake N, Tanabe K, Yoshinari M (2011) Change in zeta potential with physicochemical treatment of surface of anatase-form titania particles. J Oral Tissue Eng 9:64–70
Karakaş F, Çelik MS (2013) Mechanism of TiO2 stabilization by low molecular weight NaPAA in reference to water-borne paint suspensions. Colloids Surf A Physicochem Eng Asp 434:185–193. doi:10.1016/j.colsurfa.2013.05.051
Li LC, Tian Y (2002) Zeta potential. Encycl Pharm Technol 3020–3031:8–11. doi:10.1351/goldbook
Innocenzi P, Abdirashid MO, Guglielmi M (1994) Structure and properties of sol–gel coatings from methyltriethoxysilane and tetraethoxysilane. J Sol-Gel Sci Technol 3:47–55. doi:10.1007/BF00490148
Matsuda A, Matsuno Y, Tatsumisago M, Minami T (1998) Fine patterning and characterization of gel films derived from methyltriethoxysilane and tetraethoxysilane. J Am Ceram Soc 81:2849–2852. doi:10.1111/j.1151-2916.1998.tb02705.x
Kawai T, Tsutsumi K (1998) Reactivity of silanol groups on zeolite surfaces. Colloid Polym Sci 276:992–998. doi:10.1007/s003960050338
Fadeev AY, Mccarthy TJ (1998) Surface modification of poly(ethylene terephthalate) to prepare surfaces with silica-like reactivity. Langmuir 14:5586–5593. doi:10.1021/la980512f
Tanaka M, Sawaguchi T, Kuwahara M, Niwa O (2013) Surface modification of silicon oxide with trialkoxysilanes toward close-packed monolayer formation. Langmuir 29:6361–6368. doi:10.1021/la4009834
AZoM.com (2016) Properties: silica–silicon dioxide (SiO2). http://www.azom.com/properties.aspx?ArticleID=1114
Amils RI, Gallego JD, Sebastián JL et al (2016) Thermal conductivity of silver loaded conductive epoxy from cryogenic to ambient temperature and its application for precision cryogenic noise measurements. Cryogenics (Guildf) 76:23–28. doi:10.1016/j.cryogenics.2016.03.001
Shahil KMF, Balandin AA (2012) Graphene-multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett 12:861–867. doi:10.1021/nl203906r
Acknowledgments
This work was supported by the Energy Efficiency and Resources (No. 20122010100120) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) Grant funded by the Korea Government Ministry of Knowledge Economy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, S., Cha, D.A., Hwang, YH. et al. Adsorbent layer for adsorption heat pump prepared with the surface-modified ferroaluminophosphate particles and inorganic silica binder. J Sol-Gel Sci Technol 80, 297–305 (2016). https://doi.org/10.1007/s10971-016-4132-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4132-y