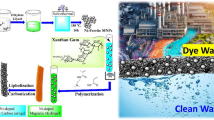
Abstract
To develop an effective and novel adsorbent material consisting of three-layered core–shell particles with magnetic and photocatalytic properties, this study has utilized a sol–gel technique to synthesize Fe3O4/SiO2/TiO2/PAM (polyacrylamide) nanocomposite (FSTP NCs)-functionalized Fe3O4/SiO2/TiO2NPs as core and PAM as shell for water purification. The surface of the TiO2 layer has been treated with silane A-174 (AA) as a coupling agent. In the final step, NPs were coated with PAM as an organic layer through radical polymerization of AA, to prepare a well-structured nanocomposite. FTIR, SEM, EDX, TEM, XRD, and VSM were applied to investigate the novel composed bonds, morphological properties of the surface and elemental analysis, core–shell structures, NPs size, samples phase and superparamagnetism of the NC and NPs, respectively. The R2 values in three models of Langmuir (0.89), Freundlich (0.84), and Dubinin-Radushkevich (0.98) were calculated and the isotherm model followed a model with the highest R2. The maximum efficiency of arsenate removal was recorded in 0.1 g concentration of adsorbent, pH 2, contact time of 700 min, and ion concentration of 50 mg/L.
Graphical abstract












Similar content being viewed by others
References
Saleh TA, Mustaqeem M, Khaled M (2022) Water treatment technologies in removing heavy metal ions from wastewater: a review. Environ Nanotechnol Monit Manag 17:100617. https://doi.org/10.1016/J.ENMM.2021.100617
Lei Y, Zhang X, Meng X, Wang Z (2022) The preparation of core-shell Fe3O4@SiO2magnetic nanoparticles with different surface carboxyl densities and their application in the removal of methylene blue. Inorg Chem Commun 139:109381. https://doi.org/10.1016/J.INOCHE.2022.109381
Tolkou AK, Kyzas GZ, Katsoyiannis IA (2022) Arsenic (V) removal from water sources by molecularly imprinted polymers (MIPs): A mini review of recent developments. Sustainability 14:5222. https://doi.org/10.3390/su14095222
Ali MAM, Alsabagh AM, Sabaa MW, El-Salamony RA, Mohamed RR, Morsi RE (2020) Polyacrylamide hybrid nanocomposites hydrogels for efficient water treatment. Iran Polym J 29:455–466. https://doi.org/10.1007/s13726-020-00810-y
Vani B, Shivakumar M, Kalyani S, Sridhar S (2021) TiO2 nanoparticles incorporated high-performance polyphenyl sulfone mixed matrix membranes for ultrafiltration of domestic greywater. Iran Polym J 30:917–934. https://doi.org/10.1007/s13726-021-00945-6
Jabbar KQ, Barzinjy AA, Hamad SM (2022) Iron oxide nanoparticles: preparation methods, functions, adsorption and coagulation/flocculation in wastewater treatment. Environ Nanotechnol MonitManag 17:100661. https://doi.org/10.1016/J.ENMM.2022.100661
Mallakpour S, Tabesh F (2021) Application of gum polysaccharide nanocomposites in the removal of industrial organic and inorganic pollutants. Handbook Polym Nanocompos Ind Appl 2021:503–528. https://doi.org/10.1016/B978-0-12-821497-8.00018-6
Zhao G, Huang X, Tang Z, Huang Q, Niu F, Wang X (2018) Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem 9:3562–3582. https://doi.org/10.1039/C8PY00484F
Bassyouni M, Abdel-Aziz MH, Zoromba MS, Abdel-Hamid SM, Drioli E (2019) A review of polymeric nanocomposite membranes for water purification. J Ind Eng Chem 73:19–46. https://doi.org/10.1016/J.JIEC.2019.01.045
Saleh TA (2021) Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies. Environ Technol Innov 24:101821. https://doi.org/10.1016/J.ETI.2021.101821
Beyene HD, Ambaye TG (2019) Application of sustainable nanocomposites for water purification process. Sustain Polym Compos Nanocompos 2019:387–412
Maio A, Gammino M, Gulino EF, Megna B, Fara P, Scaffaro R (2020) Rapid one-step fabrication of graphene oxide-decorated polycaprolactone three-dimensional templates for water treatment. ACS Appl Polym Mater 2020:4993–5005. https://doi.org/10.1021/acsapm.0c00852
Li L, Li X, Duan H, Wang X, Luo C (2014) Removal of Congo Red by magnetic mesoporous titanium dioxide–graphene oxide core-shell microspheres for water purification. Dalton Trans 43:8431. https://doi.org/10.1039/c3dt53474j
Mallakpour S, Tabesh F (2020) Fabrication technologies of layered double hydroxide polymer nanocomposites. Layered Double Hydroxide Polym Nanocompos 2020:103–155. https://doi.org/10.1016/B978-0-08-101903-0.00003-9
Rizvi MA, Moosvi SK, Jan T, Bashir S, Kumar P, Roos WD, Swart HC (2019) Dielectric, magnetic and photocatalytic activity of polypyrrole/Prussian red nanocomposite for waste water treatment applications. Polymer 163:1–12. https://doi.org/10.1016/J.POLYMER.2018.12.044
Fan J, Zhang S (2015) Facile preparation of Fe3O4/mesoporous TiO2nanoparticles shell on polystyrene beads and its effective absorption of cyanobacteria in water. J Polym Res 22:182. https://doi.org/10.1007/s10965-015-0818-z
Arumugam V, Redhi GG, Gengan RM (2016) Efficient catalytic activity of ionic liquid-supported NiFe2O4 magnetic nanoparticle doped titanium dioxide nano-composite. Int J Chem Eng Appl 7:422–427 https://doi.org/10.18178/ijcea.2016.7.6.618
Valamohammadi E, Behdarvand F, Mohammadi T, Tofighy MA, Moghiseh Z (2022) Effects of carbon nanotubes on structure, performance and properties of polymer nanocomposite membranes for water/wastewater treatment applications: a comprehensive review. Polym Bull 18:1–44. https://doi.org/10.1007/s00289-022-04635-y
Saleh TA, Parthasarathy P, Irfan M (2019) Advanced functional polymer nanocomposites and their use in water ultra-purification. Trends Environ Anal Chem 24:e00067. https://doi.org/10.1016/J.TEAC.2019.E00067
Abdollahi H, Ershad-Langroudi A, Salimi A, Rahimi A (2014) Anticorrosive coatings prepared using epoxy-silica hybrid nanocomposite materials. Ind Eng Chem Res 53:10858–10869. https://doi.org/10.1021/ie501289g
Kumar M, Dosanjh HS, Singh J, Monir K, Singh H (2020) Review on magnetic nanoferrites and their composites as alternatives in waste water treatment: synthesis, modifications and applications. Environ Sci 6:491–514. https://doi.org/10.1039/C9EW00858F
Pazokifard S, Farrokhpay S, Mirabedini M, Esfandeh M (2015) Surface treatment of TiO2 nanoparticles via sol-gel method: effect of silane type on hydrophobicity of the nanoparticles. Prog Org Coat 87:36–44. https://doi.org/10.1016/J.PORGCOAT.2015.04.021
Abid M, Ben Haj Amara A, Bechelany M (2023) Halloysite-TiO2 nanocomposites for water treatment: a review. Nanomaterials 13:1578. https://doi.org/10.3390/nano13091578
Alsaiari NS, Amari A, Katubi KM, Alzahrani FM, Rebah FB, Tahoon MA (2021) Innovative magnetite based polymeric nanocomposite for simultaneous removal of methyl orange and hexavalent chromium from water. Processes 9:576. https://doi.org/10.3390/pr9040576
Acharya R, Lenka A, Parida K (2021) Magnetite modified amino group based polymer nanocomposites towards efficient adsorptive detoxification of aqueous Cr (VI): a review. J Mol Liq 337:116487. https://doi.org/10.1016/J.MOLLIQ.2021.116487
Mudassir MA, Hussain SZ, Jilani A, Zhang H, Ansari TM, Hussain I (2019) Magnetic hierarchically macroporous emulsion-templated poly (acrylic acid)–iron oxide nanocomposite beads for water remediation. Langmuir 35:8996–9003. https://doi.org/10.1021/acs.langmuir.9b01121
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112:5073–5091. https://doi.org/10.1021/cr300133d
Falciglia PP, Gagliano E, Scandura P, Bianco C, Tosco T, Sethi R, Varvaro G, Agostinelli E, Bongiorno C, Russo A, Romano S (2022) Physico-magnetic properties and dynamics of magnetite (Fe3O4) nanoparticles (MNPs) under the effect of permanent magnetic fields in contaminated water treatment applications. Sep Purif Technol 296:121342. https://doi.org/10.1016/J.SEPPUR.2022.121342
Zhang Q, Lu D, Wang D, Yang X, Zuo P, Yang H, Fu Q, Liu Q, Jiang G (2020) Separation and tracing of anthropogenic magnetite nanoparticles in the urban atmosphere. Environ Sci Technol 54:9274–9284. https://doi.org/10.1021/acs.est.0c01841
Sosun AA, Mannan A, Ali Shah U, Zia M (2022) Removal of toxic metal ions (Ni2+ and Cd2+) from wastewater by using TOPO decorated iron oxide nanoparticles. Appl Water Sci 12:86. https://doi.org/10.1007/s13201-022-01588-5
Hong J, Xie J, Mirshahghassemi S, Lead J (2020) Metal (Cd, Cr, Ni, Pb) removal from environmentally relevant waters using polyvinylpyrrolidone-coated magnetite nanoparticles. RSC Adv 10:3266–3276. https://doi.org/10.1039/C9RA10104G
Mohammadi A, Barikani M, Barmar M (2013) Effect of surface modification of Fe3O4 nanoparticles on thermal and mechanical properties of magnetic polyurethane elastomer nanocomposites. J Mater Sci 48:7493–7502. https://doi.org/10.1007/s10853-013-7563-7
Samrot AV, Sahithya CS, Selvarani J, Purayil SK, Ponnaiah P (2021) A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Current Res Green Sustain Chem 4:100042. https://doi.org/10.1016/J.CRGSC.2020.100042
Bustamante-Torres M, Romero-Fierro D, Estrella-Nuñez J, Arcentales-Vera B, Chichande-Proaño E, Bucio E (2022) Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: a review. Polymers 14:752. https://doi.org/10.3390/polym14040752
Roca AG, Gutiérrez L, Gavilán H, Brollo ME, Veintemillas-Verdaguer S, del Puerto MM (2019) Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv Drug Deliv Rev 138:68–104. https://doi.org/10.1016/J.ADDR.2018.12.008
Raza M, Bachinger A, Zahn N, Kickelbick G (2014) Interaction and UV-stability of various organic capping agents on the surface of anatase nanoparticles. Materials 7:2890–2912. https://doi.org/10.3390/ma7042890
Salman D, Juzsakova T, Al-Mayyahi MA, Ákos R, Mohsen S, Ibrahim RI, Mohammed HD, Abdullah TA, Domokos E, Korim T (2021) Synthesis, surface modification and characterization of magnetic Fe3O4@ SiO2 core-shell nanoparticles. J Phys Conf Ser 1773:012039. https://doi.org/10.1088/1742-6596/1773/1/012039
Liu S, Yu B, Wang S, Shen Y, Cong H (2020) Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv Colloid Interface Sci 281:102165. https://doi.org/10.1016/J.CIS.2020.102165
Wang Y, Gu H (2015) Core-shell-type magnetic mesoporous silica nanocomposites for bioimaging and therapeutic agent delivery. Adv Mater 27:576–585. https://doi.org/10.1002/adma.201401124
Bhateria R, Singh R (2019) A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J Water Proc Eng 31:100845. https://doi.org/10.1016/J.JWPE.2019.100845
Cheng JP, Ma R, Li M, Wu JS, Liu F, Zhang XB (2012) Anatase nanocrystals coating on silica-coated magnetite: role of polyacrylic acid treatment and its photocatalytic properties. Chem Eng J 210:80–86. https://doi.org/10.1016/J.CEJ.2012.08.059
Jacinto MJ, Ferreira LF, Silva VC (2020) Magnetic materials for photocatalytic applications: a review. J Solgel Sci Technol 96:1–14. https://doi.org/10.1007/s10971-020-05333-9
Ershad-Langroudi A, Rabiee A (2012) A novel acrylamide-anatase hybrid nanocomposite. J Polym Res 19:9970. https://doi.org/10.1007/s10965-012-9970-x
Sadiaa M, Saqiba A, Khana J, Zahoorb M, Zekkerc I (2022) Photocatalytic degradation of methyl orange and toluidine blue using advanced oxidation method. Desalin Water Treat 262:256–265. https://doi.org/10.5004/dwt.2022.28554
Inturi SNR, Boningari T, Suidan M, Smirniotis PG (2014) Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl Catal B 144:333–342. https://doi.org/10.1016/j.apcatb.2013.07.032
Rabiee A, Ershad-Langroudi A, Zeynali ME (2015) A survey on cationic polyelectrolytes and their applications: acrylamide derivatives. Rev Chem Eng 31:56. https://doi.org/10.1515/revce-2014-0056
Maćczak P, Kaczmarek H, Ziegler-Borowska M (2020) Recent achievements in polymer bio-based flocculants for water treatment. Materials 13:3951. https://doi.org/10.3390/ma13183951
Pashapouryeganeh F, Zargar G, Rabiee A, Kadkhodaie A, Takassi MA (2022) Design and synthesis of cationic copolymer synergized with metal nanoparticles as polymeric hybrid nanocomposite for carbonate reservoir applications. Polym Bull 80:7865–7882. https://doi.org/10.1007/s00289-022-04405-w
Kokate M, Garadkar K, Gole A (2013) One pot synthesis of magnetite-silica nanocomposites: applications as tags, entrapment matrix and in water purification. J Mater Chem A 1:2022–2029. https://doi.org/10.1039/C2TA00951J
Jain R (2022) Recent advances of magnetite nanomaterials to remove arsenic from water. RSC Adv 12:32197–32209. https://doi.org/10.1039/D2RA05832D
Wang C, Yin L, Zhang L, Kang L, Wang X, Gao R (2009) Magnetic (γ-Fe2O3@SiO2)n@TiO2 functional hybrid nanoparticles with activated photocatalytic ability. J Phys Chem C 113:4008–4011. https://doi.org/10.1021/jp809835a
Shchukin DG, Ustinovich EA, Sviridov DV, Kulak AI (2004) Titanium and iron oxide-based magnetic photocatalysts for oxidation of organic compounds and sulfur dioxide. High Energy Chem 38:167–173. https://doi.org/10.1023/B:HIEC.0000027654.45001.00
Khoo YS, Lau WJ, Liang YY, Karaman M, Gürsoy M, Ismail AF (2022) Eco-friendly surface modification approach to develop thin film nanocomposite membrane with improved desalination and antifouling properties. J Adv Res 36:39–49. https://doi.org/10.1016/j.jare.2021.06.011
Fonseca FV, Silva LLS, Linhares AMF, Borges CP (2023) Current trends of nano-enhanced polymeric membranes for water and wastewater reclamation. Novel Mater Environ Remed Appl 2023:63–98. https://doi.org/10.1016/B978-0-323-91894-7.00018-9
Dadpanah A, Rabiee A, Mohammadi F, ErshadLangroudi A, Zeynali ME (2022) Preparation and characterization of AM-co-APTAC/TiO2nanocomposite for environmental applications. Polym Bull 79:1039–1055. https://doi.org/10.1007/s00289-020-03512-w
Ashraf S, Siddiqa A, Shahida S, Qaisar S (2019) Titanium-based nanocomposite materials for arsenic removal from water: a review. Heliyon 5:e01577. https://doi.org/10.1016/j.heliyon.2019.e01577
Xu J, Ju C, Sheng J, Wang F, Zhang Q, Sun G, Sun M (2013) Synthesis and characterization of magnetic nanoparticles and its application in lipase immobilization. Bull Korean Chem Soc 34:2408–2412. https://doi.org/10.5012/bkcs.2013.34.8.2408
Kittappa S, Pichiah S, Kim JR, Yoon Y, Snyder SA, Jang M (2015) Magnetised nanocomposite mesoporous silica and its application for effective removal of methylene blue from aqueous solution. Sep Purif Technol 153:67–75. https://doi.org/10.1016/J.SEPPUR.2015.08.019
Yang N, Luo ZX, Chen SC, Wu G, Wang YZ (2020) Fe3O4 nanoparticle/N-doped carbon hierarchically hollow microspheres for broadband and high-performance microwave absorption at an ultralow filler loading. ACS Appl Mater Interfaces 12:18952–18963. https://doi.org/10.1021/acsami.0c04185
Asab G, Zereffa EA, AbdoSeghne T (2020) Synthesis of silica-coated Fe3O4 nanoparticles by microemulsion method: characterization and evaluation of antimicrobial activity. Int J Biomater 2020:1–11. https://doi.org/10.1155/2020/4783612
Yuan Q, Li N, Geng W, Chi Y, Li X (2012) Preparation of magnetically recoverable Fe3O4@SiO2@meso-TiO2nanocomposites with enhanced photocatalytic ability. Mater Res Bull 47:2396–2402. https://doi.org/10.1016/j.materresbull.2012.05.031
Absalan F, Nikazar M (2016) Application of response surface methodology for optimization of water treatment by Fe3O4 /SiO2 /TiO2 core-shell nano-photocatalyst. Chem Eng Commun 203:1523–1531. https://doi.org/10.1080/00986445.2016.1218335
Mousavi SE, Younesi H, Bahramifar N, Tamunaidu P, Karimi-Maleh H (2022) A novel route to the synthesis of α-Fe3O4/SiO2/TiO2 nanocomposite from the metal-organic framework as a photocatalyst for water treatment. Chemosphere 297:133992. https://doi.org/10.1016/j.chemosphere.2022.133992
Corredor LM, Husein MM, Maini BB (2019) Impact of PAM-grafted nanoparticles on the performance of hydrolyzed polyacrylamide solutions for heavy oil recovery at different salinities. Ind Eng Chem Res 58:9888–9899. https://doi.org/10.1021/acs.iecr.9b01290
Wang H, Chao L, Wei X, Li J, Ji C, Wang B, Qi X, Hu P, Ying Y, Tian M (2019) Design of SiO2-TiO2-PAM composite flocculant with self-degrading characteristics and optimization of the flocculation process using a combination of central composite design and response surface methodology. Colloids Surf A Physicochem Eng Asp 583:123982. https://doi.org/10.1016/j.colsurfa.2019.123982
Mallakpour S, Abdolmaleki A, Tabesh F (2018) Ultrasonic-assisted manufacturing of new hydrogel nanocomposite biosorbent containing calcium carbonate nanoparticles and tragacanth gum for removal of heavy metal. Ultrason Sonochem 41:572–581. https://doi.org/10.1016/j.ultsonch.2017.10.022
Dada AO, Olalekan AP, Olatunya AM, Dada OJ (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45. https://doi.org/10.9790/5736-0313845
Mallakpour S, Tabesh F (2019) Tragacanth gum based hydrogel nanocomposites for the adsorption of methylene blue: Comparison of linear and non-linear forms of different adsorption isotherm and kinetics models. Int J Biol Macromol 133:754–766. https://doi.org/10.1016/j.ijbiomac.2019.04.129
Brion-Roby R, Gagnon J, Deschênes JS, Chabot B (2018) Development and treatment procedure of arsenic-contaminated water using a new and green chitosan sorbent: kinetic, isotherm, thermodynamic and dynamic studies. Pure Appl Chem 90:63–77. https://doi.org/10.1515/pac-2017-0305
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salahi, F., Ershad-Langroudi, A. & Didehban, K. Inorganic particles/silica/polyacrylamide nanocomposite: as a potential application in water treatment. Iran Polym J 33, 405–418 (2024). https://doi.org/10.1007/s13726-023-01263-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-023-01263-9