Abstract
Inorganic salt melts are used for the preparation of ceramics. It turns out that such ionothermal syntheses can also be employed in the chemistry of carbon. Carbon materials with improved application-relevant properties such as high surface area and large pore volume can be obtained. The way these properties are obtained strongly reminds on classic sol–gel synthesis, which displays a comparably easy approach toward such porous carbons. The central role of the solvent, i.e., the inorganic salt melt allows for variation of the chemical and morphological structure of carbon products. Interestingly, the use of inorganic salt melts may also give insights into the crystallization of carbon, if precursors are directly added to the hot melt, which additionally guarantees reorganizational dynamics to the pyrolysis intermediates.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon materials are a very important and interesting class of materials having a number of applications due to their unique chemical and physical properties, such as electrical conductivity, heat conductivity, mechanical and chemical stability. The exploration of carbon allotropes revolutionized chemistry and materials science, and there are still many other theoretical allotropic forms to discover. Even amorphous carbons, which often contain heteroatoms, referred to as heteroatom-doped carbons or carbon alloys, are very relevant. By example, such materials act as electrocatalysts with a potential to substitute the expensive platinum in fuel cells on the oxygen reduction side [1–3]. Nanostructured carbon materials comprise special and sometimes ordered morphologies and have high internal surface area and porosity. In many processes such as adsorption, separation, energy conversion and storage, this leads to high activity.
1.1 Synthetic strategies toward nanostructured carbon materials
There are a number of preparative strategies to generate nanostructured carbons. Typically such nanostructured carbons comprise high porosity, and therefore, the methods can be summarized with the term porogenesis. An author’s selection of important pathways is schematically presented in Fig. 1.
Schematic presentation of selected preparation procedures of nanostructured carbon materials. a Chemical/physical activation; b hard templating; c soft templating (SA = self-assembly) and d sol–gel process. Reprinted (adapted) from [7]
High internal surface areas can be obtained by leaching of carbon atoms by chemical reaction. For historical reasons, this method is called activation and is divided into “chemical” and “physical” activation, as either manageable leaching agent is used or the carbon is leached in a stream of reactive gas (Fig. 1a). Carbon precursors as simple and cheap as coconut shells can be used, so activated carbons can be produced at very low costs and can reach very high surface areas of up to 3000 m2/g. Disadvantageous are low yields and the top-down mode of production, which limits the control of desired chemical compositions such as the heteroatom content, but also particle shape and size.
Analogous to the pottery technique, in templating strategies, nanostructured non-carbonizable materials are used as a sacrificial mold with known structure to influence the carbonization. After removal of the structure directing mold, the replicated carbon structure remains. The so-called hard and soft templating procedures are distinguished. In the first typically inorganic nanomaterials are used and concurrently dissolved/leached in a wet-chemical step (Fig. 1b). The latter process makes use of structure directing agents such as detergents or polymeric amphiphiles that generate nanoscopic two phase systems by self-assembly (SA) (Fig. 1c). The resulting carbon is herein obtained throughout polymerization/cross-linking followed by carbonization of one of the two phases. Precursors typically are monomers of carbonizable polymers or resins like polyacrylonitrile or resorcinol [4–6]. Both templating strategies can be employed to obtain highly ordered nanostructures with high surface area and porosity. The downside is the additional costs of template preparation and removal as well as the often observed shrinkage of the aimed structure to the point of pore collapse induced by the decomposition of the soft templates already at moderate temperatures.
The sol–gel process creates a porous gel simply by agglomeration of polymerized/cross-linked primary nanoparticles (Fig. 1d). This works, e.g., by accelerated polycondensation of aqueous resorcinol formaldehyde mixtures at extreme pH conditions. The porogen is therefore simply the solvent, which makes the process quiet efficient. To transform the organic gel into a carbon gel, carbonization at high temperatures is required. Previous removal of the solvent but also thermolysis in the absence of a supporting porogen at high temperatures again leads to shrinkage and pore collapse.
Advantages of templating and the sol–gel process is the possibility of constructing a carbon material with desired chemical composition, which lead to the term of designed carbons.
2 Results
2.1 Inorganic salt melts as porogen and solvent
Can we find solvents that withstand carbonization at high temperatures?
Melts of inorganic salts (SMs) and the mixtures thereof show a wide liquidus and by example allow for efficient mass transport as a so-called flux and are used in the production of high temperature ceramics. Presumably because the melting point of common salts is high compared to the reaction onset of organic polymerization/cross-linking reactions, SMs were rarely considered a potential reaction medium for carbonization [8–10]. Additionally, precursors should be stable and soluble in or miscible with the inorganic melt to guarantee homogeneous reaction conditions. In recent years, molten ZnCl2 was employed for the synthesis of covalent triazine-based organic frameworks [11]. The crystallization of graphitic carbon nitrides was successfully obtained from a eutectic LiCl/KCl melt [12–14]. On top of that, some polymers were successfully dissolved in salt melts [15]. Solubility of organic molecules is thus given at least in few cases. In fact, some eutectics show relatively low melting points (e.g., NaCl/ZnCl2 with T m = 230 °C), so that there is the chance to find carbonizable precursors, which are stable and soluble in molten salts at this temperature.
Very interesting precursors for such “ionothermal” syntheses are organic salts, as solubility in inorganic SMs is highly probable. Specifically, it is known that ionic liquids, i.e., low-melting organic salts, with an N-heterocyclic cation and the dicyanamide anion are carbonizable and produce nitrogen-doped carbon via thermolysis under inert atmosphere [16]. Figure 2a shows the reaction scheme of carbonization of the pure ionic liquid 1-ethyl-3-methylimidazolium dicyanamide (Emim-dca). In Fig. 2b, the process of ionothermal carbonization is drafted. The archetype system comprises carbonization of the ionic liquid (herein Emim-dca) from solution in molten eutectic NaCl/ZnCl2. The eutectic inorganic salt is simply mixed with the ionic liquid under dry conditions, transferred to a ceramic crucible and calcined under inert atmosphere. The product is obtained as a powder after aqueous removal of the salt. Considering the recovery of the salt porogen, we have an efficient cyclic process [17].
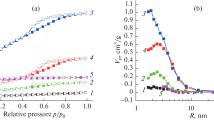
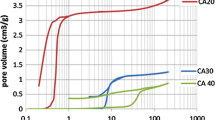
The combination of properties of organic and inorganic salts indeed leads to porous nitrogen-doped carbons with very high specific surface areas of more than 2500 m2 g−1 throughout heat treatment of the simple mixture [17, 19]. The material comprises nitrogen atoms, which according to X-ray photoelectron spectroscopy (XPS) are a mixture of pyridinic, quaternary graphitic and oxidized species (Fig. 3a). The presence of the inorganic salt leads to efficient precursor conversion with yields almost double as high (~40 %) as the carbonization of the pure ionic liquid. Depending on the chosen ratio of ionic liquid and inorganic salt, microporous carbons with moderate pore volumes or fluffy carbon aerogels (Fig. 3b) with very high pore volumes (>3 cm3 g−1 based on nitrogen physisorption measurements) are obtained [17, 20]. The carbon morphology also strongly depends on the choice of the eutectic, which apparently can be attributed to the different miscibility and viscosity. These properties point to an applicable description of the process as a sol–gel process like shown in Fig. 1d. Obtained gels are desirable for multiple applications, as the hierarchical porosity—evaluated by N2-physisorption (Fig. 3c)—with concurrent presence of micro-, meso- and macropores, are advantageous in mass transport limited processes. High-resolution transmission electron microscopy imaging (HRTEM) illustrates the reason for the high specific surface areas as even the primary nanoparticles are composed of disordered (amorphous) graphene sheets and therefore comprise microporosity (Fig. 3d, inset).
Experimental results of the ionothermal carbon for Emim-dca: NaCl/ZnCl2 = 1:13 at T = 1000 °C are shown. a High-resolution XPS spectrum at the N1 s edge, b scanning electron micrograph of the gel-like carbon (b). N2 physisorption isotherm indicating a hierarchical pore system (pore size distribution, see inset) (c), and transmission electron micrographs of the agglomerated spherical primary particles (d) and the fine structure (inset). Reprinted (adapted) from [20]
The bottom-up approach allows for variation of the chemical composition of the materials. This way the nitrogen content can be tuned, but also iron- or cobalt-based nanoparticles can be embedded in one step by simple addition of the respective salts to the reaction mixture [21]. The porogen may be removed by simple aqueous work-up, which is much easier as compared to the leaching of hard templates with sometimes hazardous chemicals. It is to mention that such nitrogen-doped carbons, because of the chemical composition, the high surface area and the hierarchical pore system show very promising performance as electrocatalysts for fuel cells [20, 21]. The applicability of such carbons is, however, not in the scope of this article, and the interested reader is referred to the original literature.
However, not only ionic liquids are suitable carbon precursors. Fischer et al. [15] extensively studied the solubility of cellulose in metal chloride hydrate melts. In fact, it was recently shown that cheap and abundant biomass can also be used for the synthesis of structurally very similar materials [22–26]. In other work, LiCl/KCl, cesium acetate or Na2CO3/K2CO3 melts were used as flux to obtain oligographene, carbon gels or active carbon [27–29].
2.2 Crystallization and self-assembly of carbon in SMs
Typically, carbon materials are obtained in solid-state reactions. Because carbonization/graphitization needs high temperatures even in the presence of catalysts, typically nonvolatile precursors are employed. Solid-state reaction, however, has the drawbacks of restricted mass transport and missing reorganizational ordering. Therefore, mostly disordered, but also heterogeneous products are obtained. This issue can be tackled with gas-phase carbonization of volatile precursors and not only fullerenes, nanotubes and graphene, but also amorphous, colloidal carbons can be prepared this way. Unfortunately, also gas-phase process have disadvantages such as low space–time yields, moderate heat transfer and temperature control. Due to the lack of any surface stabilizing agents, e.g., in spray pyrolysis spherical, non-porous materials (e.g., printing ink and conductive soot) are obtained [30]. Also here the SMs are interesting reaction media as the structure formation can be expected to be different in a liquid phase, which might act as an interface stabilizing agent.
In chemical vapor deposition, special substrates are used to direct the growth of graphene or carbon nanotubes. Carbon atoms originating from decomposed precursors intermediately form solid or liquid solutions within the substrate, and carbon formation can be understood as a recrystallization from the substrates surface.
What happens if we have a solid–liquid interface instead of the solid–gas interface in a chemical vapor deposition? Investigations using the archetype system of ionothermal carbonization in the presence of a nickel foam substrate were carried out. A mixture of Emim-dca and ZnCl2 pasted on nickel foam and treated in inert atmosphere at 900 °C gives interesting results (Fig. 4a) [31]. For comparison, blind experiments were carried out: (1) without nickel foam and (2) without ZnCl2. The product without Ni foam resembles the previously described carbon aerogels (Fig. 4b), while the pyrolysis of the pure ionic liquid on nickel foam results in a “forest” of carbon nanotubes growing from the nickel surface (Fig. 4c). In the presence of ZnCl2, however, instead of nanotubes, vertically aligned carbon nanosheets are obtained (Fig. 4d).
Synthesis scheme for the preparation of vertically aligned carbon nanosheets is shown in (a), SEM images of blind experiments Emim-dca + salt (b) and nickel foam + Emim-dca (c), as well as the products of nickel foam + salt + Emim-dca (d). “Reprinted (adapted) with permission from [31]. Copyright (2015) American Chemical Society”
Like in the case of growth of carbon nanotubes from the solid–gas interface, the nickel substrate mediates directed growth from the surface inside the melt. The presence of the molten salt porogen, however, allows for the formation of an ordered superstructure. It turns out that it is worth to study the carbon formation inside a liquid medium, as such carbon architectures, strongly bound to the Ni surface, show interesting electrochemical properties [31].
Syntheses using a substrate as a catalyst for structure formation are limited by the number of spatial degrees of freedom, which can explain the formation of (oligo)graphenes and carbon nanotubes in classical vapor deposition. A wet-chemical carbonization/graphitization could solve aforementioned problems and gives rise to interesting “new” carbon chemistry. Most of the other nanomaterials, such as metal or metal chalcogenide nanoparticles, are typically obtained in precipitations, crystallizations or self-assembly from solution. One particular interesting method to obtain nanocrystals is the so-called hot-injection technique [32]. Here precursors with low thermal stability are decomposed in a controlled and sometimes very selective fashion to enforce precipitation/crystallization. In analogy polymer, particles with desired morphology are prepared in carbon chemistry and carbonized afterward. The structure of the resulting carbon particles, however, cannot be directed, and it remains a solid-state carbonization.
But what is happening if thermal instable organic precursors are injected into hot salt melts?
If classic organic solvents like ethanol, acetonitrile or glycol are added dropwise into molten ZnCl2 at 550 °C under inert atmosphere, indeed carbon materials are obtained with sometimes surprising high yields [33, 34]. A schematic view of the synthesis is shown in Fig. 5. The composition of the obtained carbon materials can be varied by the choice of the precursor solvent. Acetonitrile, benzonitrile and pyridine after simple aqueous work-up are giving nitrogen-doped carbon, whereas DMSO after alkaline work-up leads to sulfur-doped carbon. The materials partly show very high specific surface areas and pore volumes (up to 1666 m2 g−1 and 2.8 cm3 g−1). In particular interesting are, however, the obtained morphologies (Fig. 5).
Schematic view on the ionothermal hot injection, as well as the used organic solvents as precursors and the obtained product morphologies: gel-like spherical nanoparticles (a), porous carbon sheets (b) and branched carbon nanofibers (c). Reprinted (adapted) from [34]
SEM images show that depending on the choice of the precursor solvent, three different morphologies can be obtained. First of all, we can observer the “typical” gel-like agglomerated spherical nanoparticles (Fig. 5a), which originate from solvents such as acetonitrile and benzonitrile. The second structure is composed of extended, porous layers with a thickness of ~100 nm, which, e.g., are obtained from injection of ethylene glycol (Fig. 5b). The third and most interesting structures are branched carbon nanofibers, which degree of branching and aspect ratio depends on the choice of the precursor but even more on the choice of salt melt (Fig. 5c). The interesting morphology reminds on inorganic materials, which were obtained by vectorial alignment of primary particles, i.e., by self-assembly or oriented crystallization. High-resolution TEM imaging indicated in fact that the fibers evolve from “clustering” primary sheet-like carbon nanoparticles; however, more detailed studies are necessary (Fig. 6).
HRTEM image of carbon nanofibers and scheme of the expected tectonic structure. Reprinted (adapted) from [34]
3 Conclusions
In summary, the employment of SMs as reaction media for carbonization of dissolved organic precursors, the so-called ionothermal carbonization, leads to interesting and application-relevant products and is an alternative to state-of-art synthesis procedures. It is interesting that the interaction between selected melts and the organic phase is so high that large surface areas can be stabilized, and carbon particles stay dispersed. This way the melt can perfectly act as a porogen and solvent of reaction intermediates. Instant carbonization by pyrolysis of precursors, which are added to the hot melt, is a promising way to crystallization/precipitation of carbons from the liquid state. Besides first interesting results in this direction, it is our hope that more and possibly unexpected new carbon materials will be obtained by this mode of carbonization.
References
Liu R, Wu D, Feng X, Müllen K (2010) Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew Chem 122:2619–2623
Yang W, Fellinger T-P, Antonietti M (2010) Efficient metal-free oxygen reduction in alkaline medium on high-surface-area mesoporous nitrogen-doped carbons made from ionic liquids and nucleobases. J Am Chem Soc 133:206–209
Masa J, Xia W, Muhler M, Schuhmann W (2015) On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angew Chem 54:10102–10120
Kubo S, White RJ, Tauer K, Titirici M-M (2013) Flexible coral-like carbon nanoarchitectures via a dual block copolymer-latex templating approach. Chem Mater 25:4781–4790
Chuenchom L, Kraehnert R, Smarsly BM (2012) Recent progress in soft-templating of porous carbon materials. Soft Matter 8:10801–10812
Jang J, Bae J (2005) Fabrication of mesoporous polymer using soft template method. Chem Commun 1200–1202. doi:10.1039/B416518G
Fellinger T (2015) Mit Salzschmelzen zu neuen Designerkohlen. Nachr Chem 63:979–983
Ota E, Inoue S, Horiguchi M, Otani S (1979) A novel carbonization of naphthalene and other aromatic compounds in a molten mixture of AlCl3–NaCl–KCl. Bull Chem Soc Jpn 52:3400–3406
Ota E, Otani S (1975) Carbonization of aromatic compounds in molten salt. Chem Lett 4:241–242
Nesper R, Ivantchenko A, Krumeich F (2006) Synthesis and characterization of carbon-based nanoparticles and highly magnetic nanoparticles with carbon coatings. Adv Funct Mater 16:296–305
Kuhn P, Antonietti M, Thomas A (2008) Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew Chem Int Ed 47:3450–3453
Algara-Siller G, Severin N, Chong SY, Bjorkman T, Palgrave RG et al (2014) Triazine-based graphitic carbon nitride: a two-dimensional semiconductor. Angew Chem 53:7450–7455
Bojdys MJ, Muller JO, Antonietti M, Thomas A (2008) Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chemistry 14:8177–8182
Wirnhier E, Döblinger M, Gunzelmann D, Senker J, Lotsch BV, Schnick W (2011) Poly(triazine imide) with intercalation of lithium and chloride ions [(C3N3)2(NHxLi1−x)3·LiCl]: a crystalline 2D carbon nitride network. Chem A Eur J 17:3213–3221
Fischer S, Leipner H, Thümmler K, Brendler E, Peters J (2003) Inorganic molten salts as solvents for cellulose. Cellulose 10:227–236
Paraknowitsch JP, Zhang J, Su D, Thomas A, Antonietti M (2010) Ionic liquids as precursors for nitrogen-doped graphitic carbon. Adv Mater 22:87–92
Fechler N, Fellinger TP, Antonietti M (2013) “Salt templating”: a simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv Mater 25:75–79
Paraknowitsch JP (2009) Entwicklung von Kohlenstoffmaterialien für Energieanwendungen durch gezielte Modifikation der chemischen Struktur. Universität Potsdam, Potsdam p 157
Elumeeva K, Fechler N, Fellinger T-P, Antonietti M (2014) Metal-free ionic liquid-derived electrocatalyst for high-performance oxygen reduction in acidic and alkaline electrolytes. Mater Horiz 1:588–594
Elumeeva K, Ren JW, Antonietti M, Fellinger TP (2015) High surface iron/cobalt-containing nitrogen-doped carbon aerogels as non-precious advanced electrocatalysts for oxygen reduction. Chemelectrochem 2:584–591
Graglia M, Pampel J, Hantke T, Fellinger TP, Esposito D (2016) Nitro lignin-derived nitrogen-doped carbon as an efficient and sustainable electrocatalyst for oxygen reduction. ACS Nano 10:4364–4371
Porada S, Schipper F, Aslan M, Antonietti M, Presser V, Fellinger TP (2015) Capacitive deionization using biomass-based microporous salt-templated heteroatom-doped carbons. ChemSusChem 8:1867–1874
Schipper F, Vizintin A, Ren J, Dominko R, Fellinger TP (2015) Biomass-derived heteroatom-doped carbon aerogels from a salt melt sol–gel synthesis and their performance in Li–S batteries. ChemSusChem 8:3077–3083
Ma Z, Zhang H, Yang Z, Zhang Y, Yu B, Liu Z (2014) Highly mesoporous carbons derived from biomass feedstocks templated with eutectic salt ZnCl2/KCl. J Mater Chem A 2:19324–19329
Liu X, Zhou Y, Zhou W, Li L, Huang S, Chen S (2015) Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction. Nanoscale 7:6136–6142
Pampel J, Fellinger TP (2016) Opening of bottleneck pores for the improvement of nitrogen doped carbon electrocatalysts. Adv Energy Mater 6:1502389
Liu X, Antonietti M (2014) Molten salt activation for synthesis of porous carbon nanostructures and carbon sheets. Carbon 69:460–466
Liu X, Giordano C, Antonietti M (2014) A facile molten-salt route to graphene synthesis. Small 10:193–200
Chung KK, Fechler N, Antonietti M (2014) A salt-flux synthesis of highly porous, N- and O-doped carbons from a polymer precursor and its use for high capacity/high rate supercapacitors. Adv Porous Mater 2:61–68
Voll M, Kleinschmit P (2010) Carbon, 6. Carbon Black. Ullmann’s Encyclopedia of Industrial Chemistry
Zhu J, Sakaushi K, Clavel G, Shalom M, Antonietti M, Fellinger T-P (2015) A general salt-templating method to fabricate vertically aligned graphitic carbon nanosheets and their metal carbide hybrids for superior lithium ion batteries and water splitting. J Am Chem Soc 137:5480–5485
Kwon SG, Hyeon T (2008) Colloidal chemical synthesis and formation kinetics of uniformly sized nanocrystals of metals, oxides, and chalcogenides. Acc Chem Res 41:1696–1709
Chang Y, Antonietti M, Fellinger T-P (2015) Synthese von Kohlenstoffnanostrukturen durch ionothermale Karbonisierung von gewöhnlichen Lösungsmitteln und Lösungen. Angew Chem 127:5598–5603
Chang Y, Antonietti M, Fellinger T-P (2015) Synthesis of nanostructured carbon through ionothermal carbonization of common organic solvents and solutions. Angew Chem 54:5507–5512
Acknowledgments
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fellinger, TP. Sol–gel carbons from ionothermal syntheses. J Sol-Gel Sci Technol 81, 52–58 (2017). https://doi.org/10.1007/s10971-016-4115-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4115-z