Abstract
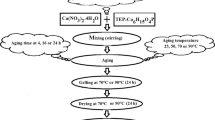
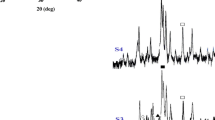
Calcium phosphate-based biomaterials are of great interest due to their use in various biomedical applications. Current preparation methods of β-tricalcium phosphate (β-TCP) require the processing of calcium phosphate precursors at high temperatures for long periods. Sol–gel-derived calcium-deficient carbonated hydroxyapatite (CHA) samples were synthesized and then aged at different times (24 and 90 h), while other freshly prepared samples were subjected to microwave (MW) radiation for 10 min in order to prepare β-TCP. All samples were calcined (at 750 °C) and then were characterized using scanning electron microscopy, Fourier transform infrared spectroscopy and X-ray diffraction. The 24-h-aged samples showed complete degradation into β-TCP and calcium pyrophosphate (CPP) phases. However, only β-TCP phase was detected in the 90-h-aged samples. Furthermore, β-TCP as the major phase was also obtained in the 10-min MW-treated unaged samples. The aging of sol–gel-derived CHA samples for 90 h had a positive effect on the conversion of CHA into β-TCP phase. Furthermore, the MW treatment of the unaged CHA samples enhanced its total conversion into β-TCP in shorter time which could be attributed to the MW irradiation-induced effect on the CHA structure.
Graphical Abstract






Similar content being viewed by others
References
Nageeb M, Nouh SR, Bergman K et al (2012) Bone engineering by biomimetic injectable hydrogel. Mol Cryst Liq Cryst 555:177–188. doi:10.1080/15421406.2012.635530
Eweida AM, Nabawi AS, Marei MK et al (2011) Mandibular reconstruction using an axially vascularized tissue-engineered construct. Ann Surg Innov Res 5:2. doi:10.1186/1750-1164-5-2
Hornberger H, Virtanen S, Boccaccini AR (2012) Biomedical coatings on magnesium alloys—a review. Acta Biomater 8:2442–2455. doi:10.1016/j.actbio.2012.04.012
Yang S, Leong KF, Du Z, Chua CK (2001) The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng 7:679–689. doi:10.1089/107632701753337645
Kumar TSS, Manjubala I, Gunasekaran J (2000) Synthesis of carbonated calcium phosphate ceramics using microwave irradiation. Biomaterials 21:1623–1629
Barakat NAM, Khalil KA, Sheikh FA et al (2008) Physiochemical characterizations of hydroxyapatite extracted from bovine bones by three different methods: extraction of biologically desirable HAp. Mater Sci Eng C 28:1381–1387. doi:10.1016/j.msec.2008.03.003
Zou Z, Lin K, Chen L, Chang J (2012) Ultrafast synthesis and characterization of carbonated hydroxyapatite nanopowders via sonochemistry-assisted microwave process. Ultrason Sonochem 19:1174–1179. doi:10.1016/j.ultsonch.2012.04.002
Sanosh KP, Chu M, Balakrishnan A, Kim TN (2009) Preparation and characterization of nano-hydroxyapatite powder using sol–gel technique. Bull Mater Sci 32:465–470
Liu D-M, Troczynski T, Tseng WJ (2002) Aging effect on the phase evolution of water-based sol–gel hydroxyapatite. Biomaterials 23:1227–1236
Wang L, Fan H, Zhang Z-Y et al (2010) Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 31:9452–9461. doi:10.1016/j.biomaterials.2010.08.036
Stiller M, Rack A, Zabler S et al (2009) Quantification of bone tissue regeneration employing beta-tricalcium phosphate by three-dimensional non-invasive synchrotron micro-tomography—a comparative examination with histomorphometry. Bone 44:619–628. doi:10.1016/j.bone.2008.10.049
Rangavittal N, Landa-Canovas AR, Gonzalez-Calbet JM, Vallet-Regi M (2000) Structural study and stability of hydroxyapatite and β-tricalcium phosphate: two important bioceramics. J Biomed Mater Res, Part A 51:660–668
Ryu H, Youn H, Hong KS et al (2002) An improvement in sintering property of β-tricalcium phosphate by addition of calcium pyrophosphate. Biomaterials 23:909–914
Matsumoto N, Yoshida K, Hashimoto K, Toda Y (2010) Preparation of beta-tricalcium phosphate powder substituted with Na/Mg ions by polymerized complex method. J Am Ceram Soc 93:3663–3670. doi:10.1111/j.1551-2916.2010.03959.x
Geng F, Tan LL, Jin XX et al (2009) The preparation, cytocompatibility, and in vitro biodegradation study of pure beta-TCP on magnesium. J Mater Sci Mater Med 20:1149–1157. doi:10.1007/s10856-008-3669-x
Lin FH, Liao CJ, Chen KS, Sun JS (1998) Preparation of high-temperature stabilized beta-tricalcium phosphate by heating deficient hydroxyapatite with Na4P2O7 × 10H2O addition. Biomaterials 19:1101–1107
Matsumoto N, Sato K, Yoshida K et al (2009) Preparation and characterization of beta-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater 5:3157–3164. doi:10.1016/j.actbio.2009.04.010
Jalota S, Tas AC, Bhaduri SB (2004) Microwave-assisted synthesis of calcium phosphate nanowhiskers. J Mater Res 19:1876–1881. doi:10.1557/JMR.2004.0230
Mahmoud MM, Folz DC, Suchicital CTA, Clark DE (2012) Crystallization of lithium disilicate glass using microwave processing. J Am Ceram Soc 95:579–585. doi:10.1111/j.1551-2916.2011.04936.x
Clarke DE, Folz DC, Folgar CE, Mahmoud MM (eds) (2005) Microwave solutions for ceramic engineers, vol 494. The American Ceramic Society, Westerville, Ohio
Clark DE, Sutton WH (1996) Microwave processing of materials. Annu Rev Mater Sci 26:299–331. doi:10.1146/annurev.ms.26.080196.001503
Agrawal D (2010) Latest global developments in microwave materials processing. Mater Res Innov 14:3–8. doi:10.1179/143307510X12599329342926
Webster TJ, Ergun C, Doremus RH et al (2001) Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials 22:1327–1333. doi:10.1016/S0142-9612(00)00285-4
Kundu PK, Waghode TS, Bahadur D, Datta D (1998) Cell culture approach to biocompatibility evaluation of unconventionally prepared hydroxyapatite. Med Biol Eng Comput 36:654–658
Murugan R, Ramakrishna S (2005) Aqueous mediated synthesis of bioresorbable nanocrystalline hydroxyapatite. J Cryst Growth 274:209–213. doi:10.1016/j.jcrysgro.2004.09.069
Huang Y, Zhou G, Zheng L et al (2012) Micro-/nano-sized hydroxyapatite directs differentiation of rat bone marrow derived mesenchymal stem cells towards an osteoblast lineage. Nanoscale 4:2484–2490. doi:10.1039/c2nr12072k
Bakan F, Laçin O, Sarac H (2013) A novel low temperature sol–gel synthesis process for thermally stable nano crystalline hydroxyapatite. Powder Technol 233:295–302. doi:10.1016/j.powtec.2012.08.030
Han J-K, Song H-Y, Saito F, Lee B-T (2006) Synthesis of high purity nano-sized hydroxyapatite powder by microwave-hydrothermal method. Mater Chem Phys 99:235–239. doi:10.1016/j.matchemphys.2005.10.017
Lee B-T, Youn M-H, Paul RK et al (2007) In situ synthesis of spherical BCP nanopowders by microwave assisted process. Mater Chem Phys 104:249–253. doi:10.1016/j.matchemphys.2007.02.009
Lak A, Mazloumi M, Mohajerani MS et al (2008) Rapid formation of mono-dispersed hydroxyapatite nanorods with narrow-size distribution via microwave irradiation. J Am Ceram Soc 91:3580–3584. doi:10.1111/j.1551-2916.2008.02690.x
Poinern G, Brundavanam R, Le XT et al (2011) Thermal and ultrasonic influence in the formation of nanometer scale hydroxyapatite bio-ceramic. Int J Nanomedicine 6:2083–2095
Mostafa NY (2005) Characterization, thermal stability and sintering of hydroxyapatite powders prepared by different routes. Mater Chem Phys 94:333–341. doi:10.1016/j.matchemphys.2005.05.011
Kuriakose TA, Kalkura SN, Palanichamy M et al (2004) Synthesis of stoichiometric nano crystalline hydroxyapatite by ethanol-based sol–gel technique at low temperature. J Cryst Growth 263:517–523. doi:10.1016/j.jcrysgro.2003.11.057
Pattanayak DK, Dash R, Prasad RC et al (2007) Synthesis and sintered properties evaluation of calcium phosphate ceramics. Mater Sci Eng C 27:684–690. doi:10.1016/j.msec.2006.06.021
Koutsopoulos S (2002) Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods. J Biomed Mater Res 62:600–612. doi:10.1002/jbm.10280
Kalita SJ, Verma S (2010) Nanocrystalline hydroxyapatite bioceramic using microwave radiation: synthesis and characterization. Mater Sci Eng C 30:295–303. doi:10.1016/j.msec.2009.11.007
Anee TK, Ashok M, Palanichamy M, Kalkura SN (2003) A novel technique to synthesize hydroxyapatite at low temperature. Mater Chem Phys 80:725–730. doi:10.1016/S0254-0584(03)00116-0
Nazir R, Iqbal N, Khan AS et al (2012) Rapid synthesis of thermally stable hydroxyapaptite. Ceram Int 38:457
Sarig S, Kahana F (2002) Rapid formation of nanocrystalline apatite. J Cryst Growth 237–239:55–59. doi:10.1016/S0022-0248(01)01850-4
Tõnsuaadu K, Gross KA, Plūduma L, Veiderma M (2012) A review on the thermal stability of calcium apatites. J Therm Anal Calorim 110:647–659. doi:10.1007/s10973-011-1877-y
Ji H, Marquis PM (1991) Modification of hydroxyapatite during transmission electron microscopy. J Mater Sci Lett 10:132–134. doi:10.1007/BF02352825
Ivanova TI, Frank-Kamenetskaya OV, Kol’tsov AB, Ugolkov VL (2001) Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal decomposition. J Solid State Chem 160:340–349. doi:10.1006/jssc.2000.9238
Cihlar J, Buchal A, Trunec M (1999) Kinetics of thermal decomposition of hydroxyapatite bioceramics. J Mater Sci 34:6121–6131
Nicolopoulos S, Gonzalez-Calbet JM, Alonso MP et al (1995) Characterization by TEM of local crystalline changes during irradiation damage of hydroxyapatite compounds. J Solid State Chem 116:265–274
Brrs EF, Hutchison JL, Senger B et al (1991) HREM study of irradiation damage in human dental enamel crystals. Ultramicroscopy 35:305–322
Suchanek WL, Shuk P, Byrappa K et al (2002) Mechanochemical–hydrothermal synthesis of carbonated apatite powders at room temperature. Biomaterials 23:699–710. doi:10.1016/S0142-9612(01)00158-2
Layrolle P, Ito A, Tateishi T (1998) Sol–gel synthesis of amorphous calcium phosphate and sintering into microporous hydroxyapatite bioceramics. J Am Ceram Soc 81:1421–1428
Kee CC, Ismail H, Mohd Noor AF (2013) Effect of synthesis technique and carbonate content on the crystallinity and morphology of carbonated hydroxyapatite. J Mater Sci Technol 29:761–764. doi:10.1016/j.jmst.2013.05.016
Wagner DE, Eisenmann KM, Nestor-kalinoski AL, Bhaduri SB (2013) A microwave-assisted solution combustion synthesis to produce europium-doped calcium phosphate nanowhiskers for bioimaging applications. Acta Biomater 9:8422–8432. doi:10.1016/j.actbio.2013.05.033
Guha A, Nayar S, Thatoi HN (2010) Microwave irradiation enhances kinetics of the biomimetic process of hydroxyapatite nanocomposites. Bioinspiration Biomim. doi:10.1088/1748-3182/5/2/024001
Acknowledgments
The authors would like to acknowledge the Egyptian Science and Technology Development Fund (STDF) for funding the current study through the Project #6118, STDF-STF agreement. The authors would like to dedicate this work for the soul of Prof. Moustafa Fakhry Khalil, Professor of Dental Biomaterials, Faculty of Dentistry, Alexandria University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, M.N., Mahmoud, M.M., El-Fattah, A.A. et al. Microwave rapid conversion of sol–gel-derived hydroxyapatite into β-tricalcium phosphate. J Sol-Gel Sci Technol 76, 74–81 (2015). https://doi.org/10.1007/s10971-015-3753-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3753-x