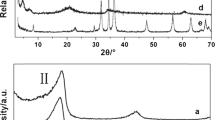
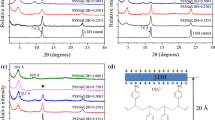
Abstract
Surfactant-intercalated MgFe-layered double hydroxides (MgFe-LDHs) were successfully synthesized via one-step self-assembly of the surfactants (sodium dodecyl sulfate, 1-hexadecane sulfate, and sodium dodecyl benzene sulfonate) and the LDH precursors without avoiding dissolved CO3 2−. As a control, p-toluene sulfonic acid was used to further study the functions of surfactants. The detailed characterization of the surfactant intercalated MgFe-LDHs and their intermediates confirm that the basal spacing changes of the formed LDHs derive from the release of surfactants out of LDH interlayers or the adsorption of surfactants from the solution in the reaction. Besides, the Mg/Fe ratio of the LDH sheets increases with the reaction and the corresponding ionic exchange capacity (IEC) of the MgFe-LDHs decreases. The final surfactant intercalated MgFe-LDH particles are the mixture of MgFe-LDH sheets with different composition and IEC, which can be the basic principle of LDH preparation for different applications. Also the Mg/Fe ratio of the surfactant intercalated MgFe-LDHs decreases with the increase of molecular length of surfactants used.






Similar content being viewed by others
References
Guo XX, Zhang FZ, Evans D, Duan GX (2010) Chem Commun 46:5197–5210
Zhao MQ, Zhang Q, Huang JQ, Wei F (2012) Adv Funct Mater 22:675–894
Zumreoglu-Karan B, Ay AN (2012) Chem Pap 66:1–10
Chen CP, Gunawan P, Xu R (2011) J Mater Chem 21:1218–1225
Koilraj P, Srinivasan K (2011) Ind Eng Chem Res 50:6943–6951
Goh KH, Lim TT, Dong ZL (2008) Water Res 42:1343–1368
Chen H, Ling QD, Zhang WG, Lin ZH (2012) Chem Eng J 185:358–365
Ladewig K, Xu ZP, Lu GQ (2009) Expert Opin Drug Del 6:907–922
Xu ZP, Zhang J, Adebajo MO, Zhang H, Zhou CH (2011) Appl Clay Sci 53:139–150
Qiu L, Chen W, Qu B (2006) Polymer 47:922–930
Matusinovic Z, Wilkie CA (2012) J Mater Chem 22:18701–18704
Ding P, Chen W, Qu BJ (2006) Prog Nat Sci 16:573–579
Zhang H, Wen X, Wang Y (2007) J Solid State Chem 180:1636–1647
Moyo L, Focke WW, Labuschagne FJWJ, Verryn S (2012) Mol Cryst Liq Cryst 555:51–64
Yilmaz C, Unal U, Acar HY (2012) J Solid State Chem 187:295–299
He J, Wei M, Li B, Kang Y, Evans DG, Duan X (2006) Layered double hydroxides. Springer, New York
Kameda T, Tsuchiya Y, Yamazaki T, Yoshioka T (2009) Solid State Sci 11:2060–2064
Wang DY, Costa FR, Vyalikh A, Leuteritz A, Scheler U, Jehnichen D, Wagenknecht U, Aussler LH, Heinrich G (2009) Chem Mater 21:4490–4497
Chen W, Qu BJ (2005) Polym. Degrad. Stabil. 90:162–166
Li PG, Lv FZ, Xu ZX, Qi GG, Zhang YH (2013) J Mater Sci 48:5437–5446
Chen W, Qu BJ (2005) Polym Degrad Stabil 90:162–166
Ding YY, Gui Z, Zhu JX, Hu Y, Wang ZZ (2008) Mater Res Bull 43:3212–3220
You YW, Zhao HT, Vance GF (2002) Colloid Surf A Physicochem Eng Asp 205:161–172
Taviot-Gueho C, Feng Y, Faour A, Leroux F (2010) Dalton Trans 39:5994–6005
Zhang H, Qi R, Duan X (2002) Chin J Inorg Chem 18:833–838
Acknowledgments
This study was financially supported by the Fundamental research Funds for the Central Universities (2010ZY46, 53200959617), special fund of Co-construction of Beijing Education committee and City University of Hong Kong Strategic research Grant(SRG,7008009).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lv, F., Zhang, R., Xu, L. et al. Composition and ionic change capacity variation of surfactant-intercalated MgFe-layered double hydroxides in the one step synthesis. J Sol-Gel Sci Technol 69, 26–32 (2014). https://doi.org/10.1007/s10971-013-3180-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3180-9