Abstract
45Ti exhibits favorable decay properties for positron emission tomography (PET) imaging and can be easily produced by the bombardment of natural scandium (Sc) by protons using the 45Sc(p,n)45Ti nuclear reaction. However, separation of 45Ti from irradiated Sc targets is arduous due to the hydrolytic instability of Ti(IV) complexes, making it a significant bottleneck for routine application of this radionuclide. In the present work, we describe the development and optimization of an ion chromatographic separation method based on trapping of 45Ti on a hydroxamate-functionalized chelating resin and subsequent elution with oxalic acid at pH = 2.8. Under optimized conditions, this method enabled 45Ti-recovery of 61 ± 8% within 7 min. Sc contamination in scaled-up experiments was found to be only 3.0 ± 1.8 µg/mL. The resulting 45Ti-solution was directly used for complexation with CDTA as a model chelator, affording the corresponding [45Ti]Ti(cdta) complex with a radiochemical conversion of 73 ± 3%. Conclusively, this promising method could be transferred to automated synthesis modules and should enable the preparation of 45Ti-labeled compounds for PET imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been growing interest in the use of non-standard radionuclides for advanced medical applications. This emerging trend is partly rooted in increasing demand for long-lived radionuclides for positron emission tomography (PET) imaging of slow (patho)physiological or biodistribution processes. In addition, significant progress in the application of radiometals for endotherapeutic purposes has spurred the need for novel metal-based PET isotopes that can be utilized in the framework of theranostic approaches [1,2,3,4,5,6,7].
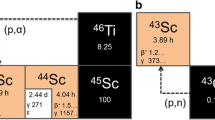
Among the available non-standard PET radionuclides, titanium-45 (45Ti) stands out due to its favorable decay properties (T½ = 3.1 h, Iβ+ = 85%, Eβ+,max = 1.04 MeV). Thus, compared to other radiometals (e.g., gallium-68, scandium-43/44 or copper-61/64 [8,9,10,11]), 45Ti exhibits a low β+-energy and negligible γ-radiation [11], which results in superior PET images and makes it a prime candidate for labeling of peptides and other biomolecules [12]. However, the fast hydrolysis of Ti(IV) complexes remains a significant impediment to the routine utilization of this radionuclide, since it hampers isolation of 45Ti from the target material and complicates the synthesis of stable radiocomplexes.
Efficient separation of 45Ti from irradiated Sc has been addressed by several working groups in the past. To assess the effectiveness of the method, different factors have to be considered like duration of the separation, the purity of 45Ti, and the simplicity of handling the high levels of radioactivity involved. Table 1 provides a comprehensive overview of various separation techniques as described in the literature.[13,14,15,16,17,18,19,20]. The highest 45Ti recovery and lowest amount of Sc impurities was reported for liquid–liquid extraction by Pedersen et al. [13]. In this method, a solvent mixture of guaiacol/anisole was applied to extract 45Ti from a hydrochloric acid solution using a dedicated in-flow liquid–liquid extraction system. The method relies on the utilization of specialized membrane filters and the application of solvents with a high-boiling point, which limits the practical applicability of this approach for automated tracer syntheses.
More recently, the thermochromatographic separation of 45Ti from Sc targets was investigated in more detail [14, 18] Thermochromatography enables isolation of the radionuclide in the form of well characterized no-carrier-added (n.c.a.) [45Ti]TiCl4 [14]. However, the air sensitivity of [45Ti]TiCl4, the time-consuming separation process as well as the rather arduous setup have prevented broad implementation of this procedure for routine tracer production.
In addition, several methods based on ion exchange chromatography have been reported in the literature [15,16,17,18,19,20,21,22], but their practical application is hampered by long separation times, poor availability of the necessary stationary phases, a need for large amounts of solvents and/or the formation of non-reactive 45Ti species that require additional processing before the radiolabeling step.
The aim of the present work was to establish a rapid chromatographic separation method which is less challenging and provides n.c.a. 45Ti in a chemical form that can be directly used for radiolabeling. Encouraged by the results of Radchenko et al. [23] on the production of a 44Sc/44Ti-generator with hydroxamate-functionalized ZR Resin™, we investigated the use of this resin to isolate 45Ti from bulk scandium targets. Originally ZR Resin™ was developed for 89Zr/Y separations but it also shows high selectivity for titanium over scandium [24]. Thus, according to Radchenko et al. [23], the distribution coefficients (Kd) of Sc and Ti on this resin in hydrochloric acid (0.1–10 M) amounted to less than 3 and more than 1000, respectively. To this end, a separation method was developed and optimized with regard to 45Ti retention on the resin, washing steps and elution conditions. In addition, the solution with 45Ti after separation was subsequently used for proof-of-principle radiolabeling experiments with CDTA as a model chelator.
Experimental
Radionuclide production
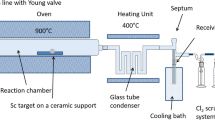
45Ti was produced via the 45Sc(p,n)45Ti nuclear reaction in high yields (337 MBq/µA*h) [11] by irradiation of a natural scandium target (330 ± 30 mg) in a copper target holder [15] with 16.9 MeV protons (2 μA for 30 min) at the Baby Cyclotron BC1710 (INM-5; Forschungszentrum Jülich). To minimize coproduction of 44Sc (T1/2: 3.9 h) [11], a 250 µm Cu foil was used to degrade the proton energy to approximately 12 MeV. During the optimization studies, some irradiations were performed without the degrader foil to enable the formation of 44Sc via the (p,pn)-process to monitor the separation and determination of radiochemical purity of 45Ti from Sc.
Ion chromatographic separation
Based on the work of Radchenko et al. [9], commercially available ZR Resin™ (Triskem, France) was selected as the stationary chromatographic phase for the separation experiments. Accordingly, Chromabond® columns were loaded with different amounts of the resin and preconditioned with 10 M HCl as described in detail in Online Appendix Sect. 3.1. The irradiated scandium target was then dissolved in 5 mL 10 M HCl. The resulting solution was diluted to 20 mL using 10 M HCl and divided into 1 mL aliquots, which were loaded onto the preconditioned columns. Unless noted otherwise, each column was washed with 5 mL 10 M HCl and an equal volume of type 1 ultra-pure water (MQ H2O) before the 45Ti was eluted with 2.5 mL of the respective elution solution (Fig. 1). For scaled-up experiments under optimized conditions, the complete target solution (5 mL) thereof were loaded onto the preconditioned columns without prior dilution. A detailed description of the experimental procedures is provided in the Online Appendix Sect. 3.2–3.8.
Retention of 45Ti
To examine the dependence of 45Ti retention on the amount of stationary phase, columns were filled with 66 ± 6 mg, 130 ± 6 mg or 280 ± 6 mg of the ZR Resin™ (n = 3 per condition). Each column was then loaded with an 1 mL aliquot of the target solution, and the percentage of retained 45Ti was determined.
Recovery of 45Ti in dependence of elution agent
For elution of 45Ti from the ZR Resin™, several weak complexing agents like hydrogen peroxide (H2O2), oxalic acid or citric acid in water or mixtures of water and organic solvents were evaluated. (see Online Appendix 3.3).
Recovery of 45Ti in dependence of pH-value
As the complexation of metal ions can be strongly affected by the pH-value, the elution efficiency of oxalic acid in concentrations ranging from 0.01 to 0.1 M was analyzed at different pH-values (Online Appendix 3.4 Table 2).
Furthermore, the influence of organic solvents on the elution efficiency was evaluated to facilitate transchelation with poorly water-soluble ligands and accelerate removal of the solvent during isolation of the resulting 45Ti-complexes. Therefore, a 0.1 M oxalic acid solution containing 20% MeOH was used.
Radiochemical purity
To assess and minimize the content of Sc in the final product, irradiations were performed without a Cu-degrader foil to produce both 45Ti and 44Sc. The target was then processed with 10 M HCl as described above, aliquots of the resulting target solution were loaded on different columns. Either 45Ti was eluted subsequently with 2.5 mL of 0.1 M oxalic acid or different washing steps were carried out. Washing solutions were 5 mL of water, 10 mL or 15 mL of 10 m HCl followed by an equal amount of water. Finally, the columns were eluted with 0.1 m oxalic acid (pH = 2.8) and the 45Ti: 44Sc ratio in the eluent was determined and compared with the ratio in the original target solution.
Batch experiments
To further determine the amount of Sc-contamination, scaled-up separation experiments under optimized conditions (130 mg ZR Resin™, 5 mL volume of wash solutions, elution with 0.1 M oxalic acid at pH = 2.8) were performed with non-radioactive Sc (350 mg) dissolved in 5 mL 10 m HCl. The solutions obtained by elution of the columns were analyzed by ICP-MS.
Additional experiments under optimized conditions were performed with 1 mL aliquots of the target solution obtained by dissolution of an irradiated Sc target in 5 mL 10 M HCL (6–35 MBq per aliquot).
Finally, to assess the suitability of the method for application in the routine production of radiopharmaceuticals, scaled-up separation experiments under the optimized conditions were performed with the entire target solution obtained by dissolution of an irradiated Sc target in 5 mL 10 M HCL (Sc: 330 ± 30 mg, 45Ti: 100–180 MBq).
Elution profile
To determine the elution profile for 45Ti, experiments under the optimized conditions were performed with aliquots (1 mL) of the target solution (21–29 MBq) and the eluate was collected in 0.5 mL fractions. The elution profile for Sc was obtained in a similar manner by using the non-radioactive Sc solution from the Batch experiment.
Complexation of 45Ti with CDTA
To demonstrate the suitability of the isolated 45Ti for radiolabeling, a proof-of-principle study was performed with 1,2-cyclohexanedinitrilotetraacetic acid (CDTA) as a model chelator. To this end, CDTA was directly added to the 45Ti solution obtained after elution with either MeCN/0.65 M H2O2 or 0.1 M oxalic acid. Radiochemical conversions (RCCs), defined as the reaction efficiency by measuring the transformation of components in a crude reaction mixture at a given time [25], were compared with those obtained with n.c.a. [45Ti]TiCl4 separated by thermochromatography [14]. Details on reaction conditions and reference compound are provided in Online Appendix 4.
Results and discussion
Retention of 45Ti
As illustrated in Fig. 2, increasing the amount of ZR Resin™ from 66 ± 6 mg to 130 ± 6 mg improved 45Ti retention from 79.7 ± 5.5% to 92.5 ± 1.7%. Due to the high standard deviation observed when the amount was further increased to 280 ± 6 mg (91.6 ± 16.1%), all subsequent experiments were performed with columns containing 130 mg of the stationary phase.
Recovery of 45Ti in dependence of elution agent
Highest recoveries of ~ 82% were observed when a mixture of acetonitrile (MeCN) and 0.65 M H2O2 was used as eluent (Fig. 3). However, this was most likely related to partial elution of the hydroxamate functional groups from the resin due to the high percentage of organic solvent. Elution with pure MeCN resulted in ~ 72% recovery as well. The degradation of the resin was indicated by insoluble components in the eluate.
Among the aqueous elution solutions, 0.5 M oxalic acid showed the best efficiency and eluted around 59% of the 45Ti from the column. Based on this finding and the fact that elution with MeCN-containing solutions proved to hamper subsequent transchelation with other ligands (see Section Complexation of 45Ti with CDTA), oxalic acid was chosen as the eluent of choice for further studies.
Recovery of 45Ti in dependence of pH-value
The results are summarzied in Fig. 4 and showed that the elution efficiency of 0.01 M oxalic acid buffered at either acidic (0.1 ammonium formate, pH = 3.2) or slightly basic (1.0 M sodium phosphate, pH = 7.9) pH-values was insufficient (< 6% recovery). In contrast, elution with unbuffered 0.1 M oxalic acid (pH = 1.3) provided a moderate recovery of 28.9 ± 6.0%, while the elution efficiency decreased when higher pH-values of the buffer solution were applied. However, a higher elution efficiency was observed for 0.05 M oxalic acid solutions, buffered with sodium phosphate at pH-values between 2.6 and 2.8 (36.2 ± 4.6% and 33.4 ± 14.7% recovery). Concentration enhancement of oxalic acid from 0.05 to 0.1 M increased the recovery of 45Ti almost two-fold (to 65.2 ± 1.2%).
In contrast, doubling the oxalic acid concentration once more to 0.2 M (66.6 ± 6.1) showed no additional effect on 45Ti recovery, as illustrated in Fig. 5.
45Ti recovery with 0.1 M oxalic acid in 20% MeOH / phosphate buffer at pH = 2.8 amounted to roughly 60% and was comparable to the recovery observed without MeOH (Fig. 5). This suggests that addition of MeOH has no negative effects on the elution efficiency of oxalic acid solutions.
Radiochemical purity
Washing with 5 mL 10 M HCl and 5 mL water resulted in an increase of the 45Ti/44Sc ratio to 4000 ± 300. When the volume of the washing solutions was increased from 5 to 10 or 15 mL, the 45Ti/44Sc-ratio in the eluent showed a progressive decline (Fig. 6), suggesting that higher volumes of washing solutions were counter-productive since 45Ti was also co-eluted. As a consequence, 5 mL 10 M HCl and water was considered as the optimal volume for the washing steps in subsequent experiments.
Batch experiments
The ICP-MS analysis of the eluate indicated an average Sc contamination of 3.0 ± 1.8 µg/mL (for details see Online Appendix 3.7 A).
Additional experiments under optimized conditions resulted in a decay corrected (d.c.) 45Ti recovery of 69 ± 10% (n = 24) (for details see Online Appendix Sect. 3.7 B).
The scaled-up separation experiments under the optimized conditions with the entire target solution showed an average separation time of 7 min and a decay corrected 45Ti recovery of 61 ± 8% (n = 9) (for details see Online Appendix Sect. 3.7 C).
Elution profiles
Figure 7 shows the elution profiles for 45Ti with 0.1 M oxalic acid at pH = 2.8 (A). With 0.1 M oxalic acid, the largest portion of 45Ti was obtained in the first four 0.5 mL fractions. For comparison, the elution profile for Sc with 0.1 M oxalic acid (B), which revealed that the major portion eluted with the second 0.5 mL fraction, is also shown.
Complexation of 45Ti with CDTA
RCCs of 92 ± 2% obtained with [45Ti]TiCl4 by thermochromatography were slightly higher in comparison to radiolabeling with eluted 45Ti in 0.1 M oxalic acid with RCCs of 73 ± 3. In contrast, radiolabeling reactions with the 45Ti solution obtained by elution with MeCN/0.65 M H2O2 afforded much lower RCCs of only 9 ± 6% (presumably due to partial elution of the hydroxamate functional groups from the ZR Resin™).
Conclusions
In this work, a method for the separation of 45Ti from irradiated Sc targets based on column chromatography has been developed and optimized. Using hydroxamate-functionalized ZR Resin™, 45Ti was recovered in yields of 61 ± 8% within 7 min resulting in an overall time of 15 min for the whole target processing. Contamination of 45Ti with other metals can hamper the radiolabeling process and necessitate higher chelator/precursor amounts are necessary. The optimized separation process developed in the present work decreased the Sc contamination from 70 mg/mL to 3.0 ± 1.8 µg/mL. Additionally, the toxicity of the metal has to be taken into account for in vivo applications. Given that Sscandium is reported to be a non-toxic element (LD50 > 400 mg/kg) [26], no adverse effects of the remaining Sc are anticipated. Subsequent complexation of 45Ti with CDTA afforded [45Ti]Ti(cdta) in RCCs of 73 ± 3%. In terms of its simplicity and short duration, the reported approach is advantageous in comparison with other methods (Table 1), since it is amenable to automation and applicable for the preparation of 45Ti-labeled compounds. The final aim of this separation technique wass to provide at least 50 MBq for in vivo application. Thus, the activities obtained in this study estimated from a comparison of the amount of 44Sc [27, 28] are already sufficient for a single PET examination.
References
Kostelnik TI, Orvig C (2019) Radioactive main group and rare earth metals for imaging and therapy. Chem Rev 119:902–956. https://doi.org/10.1021/acs.chemrev.8b00294
Roesch F, Martin M (2023) Radiometal-theranostics: the first 20 years*. J Radioanal Nucl Chem 332:1557–1576. https://doi.org/10.1007/s10967-022-08624-3
Welch MJ, Laforest R, Lewis JS (2006) Production of non-standard PET radionuclides and the application of radiopharmaceuticals labeled with these nuclides. In: Schubiger PA, Lehmann L, Friebe M (eds) PET chemistry the driving force in molecular imaging. Springer, Heidelberg, pp 159–182
Qaim SM, Spahn I (2018) Development of novel radionuclides for medical applications. J Labelled Comp Radiopharm 61:126–140. https://doi.org/10.1002/jlcr.3578
Qaim SM, Scholten B, Neumaier B (2018) New developments in the production of theranostic pairs of radionuclides. J Radioanal Nucl Chem 318:1493–1509. https://doi.org/10.1007/s10967-018-6238-x
Qaim SM (2008) Decay data and production yields of some non-standard positron emitters used in PET. Q J Nucl Med Mol Imaging 52:111–20
Notni J, Wester HJ (2018) Re-thinking the role of radiometal isotopes: towards a future concept for theranostic radiopharmaceuticals. J Labelled Comp Radiopharm 61:141–153. https://doi.org/10.1002/jlcr.3582
Martiniova L, De Palatis L, Etchebehere E, Ravizzini G (2016) Gallium-68 in medical imaging. Curr Radiopharm 9:187–207. https://doi.org/10.2174/1874471009666161028150
Walczak R, Krajewski S, Szkliniarz K et al (2015) Cyclotron production of 43Sc for PET imaging. EJNMMI Phys 2:1–10. https://doi.org/10.1186/s40658-015-0136-x
Domnanich KA, Eichler R, Müller C et al (2017) Production and separation of 43Sc for radiopharmaceutical purposes. EJNMMI Radiopharm Chem 2:1–17. https://doi.org/10.1186/s41181-017-0033-9
Qaim SM (2011) Development of novel positron emitters for medical applications: nuclear and radiochemical aspects. J Radiochim Acta 99:611–625. https://doi.org/10.1524/ract.2011.1870
Costa P, Metello L, Alves F, Duarte Naia M (2018) Cyclotron production of unconventional radionuclides for PET imaging: the example of Titanium-45 and its applications. Instruments 2:8. https://doi.org/10.3390/instruments2020008
Pedersen KS, Imbrogno J, Fonslet J et al (2018) Liquid-liquid extraction in flow of the radioisotope titanium-45 for positron emission tomography applications. React Chem Eng 3:898–904. https://doi.org/10.1039/c8re00175h
Giesen K, Spahn I, Neumaier B (2020) Thermochromatographic separation of 45Ti and subsequent radiosynthesis of [45Ti]salan. J Radioanal Nucl Chem 326:1281–1287. https://doi.org/10.1007/s10967-020-07376-2
Chen F, Valdovinos HF, Hernandez R et al (2017) Intrinsic radiolabeling of Titanium-45 using mesoporous silica nanoparticles. Acta Pharmacol Sin 38:907–913. https://doi.org/10.1038/aps.2017.1
Severin GW, Nielsen CH, Jensen AI et al (2015) Bringing radiotracing to titanium-based antineoplastics: solid phase radiosynthesis, PET and ex vivo evaluation of antitumor agent [45Ti](salan)Ti(dipic). J Med Chem 58:7591–7595. https://doi.org/10.1021/acs.jmedchem.5b01167
Gagnon K, Severin GW, Barnhart TE et al (2012) 45Ti extraction using hydroxamate resin. In: AIP Conference Proceedings. pp 211–214
Chaple IF, Thiele K, Thaggard G et al (2020) Optimized methods for production and purification of Titanium-45. Appl Radiat Isot 166:109398. https://doi.org/10.1016/j.apradiso.2020.109398
Vavere AL, Jones LA, Mccarthy TJ et al (2001) Preparation, biodistribution, and micropet imaging of 45Ti-transferrin. J Labelled Comp Radiopharm 44:793–795. https://doi.org/10.1002/jlcr.25804401279
Koller AJ, Wang L, Deluca M et al (2023) De novo approaches to the solid-phase separation of Titanium(IV) and Scandium(III): translating speciation data to selective on-bead chelation toward applications in nuclear medicine. Inorg Chem. https://doi.org/10.1021/acs.inorgchem.3c01493
Chaple IF, Lapi SE (2018) Production and use of the first-row transition metal PET radionuclides 43,44Sc, 52Mn, and 45Ti. J Nucl Med 59:1655–1659. https://doi.org/10.2967/jnumed.118.213264
Vavere AL, Welch MJ (2005) Preparation, biodistribution, and small animal PET of 45Ti- transferrin. J Nucl Med 46:683–690
Radchenko V, Meyer CAL, Engle JW et al (2016) Separation of 44Ti from proton irradiated scandium by using solid-phase extraction chromatography and design of 44Ti/44Sc generator system. J Chromatogr A 1477:39–46. https://doi.org/10.1016/j.chroma.2016.11.047
Triskem PRODUCT SHEET ZR Resin. https://www.triskem-international.com/scripts/files/61eef0f5e9fa69.45400285/PS_ZR-Resin_EN_210908.pdf. Accessed 23 Aug 2023
Herth MM, Ametamey S, Antuganov D et al (2021) On the consensus nomenclature rules for radiopharmaceutical chemistry – Reconsideration of radiochemical conversion. Nucl Med Biol 93:19–21. https://doi.org/10.1016/j.nucmedbio.2020.11.003
Weng W, Biesiekierski A, Li Y et al (2021) A review of the physiological impact of rare earth elements and their uses in biomedical Mg alloys. Acta Biomater 130:80–97. https://doi.org/10.1016/j.actbio.2021.06.004
Eppard E, de la Fuente A, Benešová M et al (2017) Clinical translation and first in-human use of [44 Sc]Sc-PSMA-617 for PET imaging of metastasized castrate-resistant prostate cancer. Theranostics 7:4359–4369. https://doi.org/10.7150/thno.20586
Rösch F, Baum RP (2011) Generator-based PET radiopharmaceuticals for molecular imaging of tumours: on the way to THERANOSTICS. Dalton Trans 40:6104–6111. https://doi.org/10.1039/c0dt01504k
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article. The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strecker, J., Wachten, T., Neumaier, B. et al. Radiochemical isolation of 45Ti using ion chromatography. J Radioanal Nucl Chem (2024). https://doi.org/10.1007/s10967-023-09270-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10967-023-09270-z