Abstract
A novel 99mTc generator was proposed herein using nanoparticles consisting of natural Mo and an alumina column for 99mTc scintigraphy. 99mTc was obtained from 99Mo decay produced via the irradiation of nanoparticles consisting of natural Mo with bremsstrahlung γ-rays using a 30 MeV electron beam. The irradiated nanoparticles were placed in an alumina column, and the 99mTc daughter from 99Mo decay was separated by the removal of the ion-exchanged water or saline. This separation procedure was repeated at regular intervals for one week. The maximum separation yield of 99mTc was 14.4 ± 0.7%, which is sufficiently high to produce 99mTc suitable for medical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tc-99 m is used in nuclear medicine to diagnose diseases of the bone, heart, lung, liver, and kidney. In particular, 99mTc-methylene diphosphonic acid (MDP) [1] and 99mTc-hydroxy methylene phosphonic acid (HMDP) [2] are commonly used to detect bone disease with an annual use of about 1.6 × 108 MBq (Japan Isotope Society statistical data 2020) [3] every year in Japan. Moreover, 99mTc-human serum albumin (HAS)-diethylene triamine pentaacetic acid [4] and 99mTc-macro-aggregated human serum albumin (MAA) [5] are used to treat heart and lung diseases at consumption rates with an annual use of about 7.6 × 107 MBq. In addition, 4.4 × 107 MBq is used for other purposes. Therefore, a total of 2.8 × 108 MBq of 99mTc is used every year for nuclear medicine-based diagnoses. The global consumption of 99Mo in 2016 was estimated at approximately 1.7 × 1010 MBq, half of which was consumed in the United States [6].
Currently, 99mTc is supplied by a highly compact generator with carrier-free 99Mo as the parent nuclide adsorbed onto an alumina column [7]. Mo-99 is produced in a nuclear reactor [8] and chemically separated from the fission products of uranium. However, supply shortages of 99Mo/99mTc are emerging as an increasingly common issue because of reactor malfunctions and international transportation accidents and backlogs. Furthermore, it is likely that the performance of nuclear reactors will deteriorate over time, resulting in additional supply shortages [9]. Many reports [10,11,12] have proposed the replacement of aging nuclear reactors for the production of 99Mo.
99Mo production through 98Mo(n, γ)99Mo [13], 100Mo (p, d)99Mo [14], and 100Mo (γ, n)99Mo reactions [15, 16] using an accelerator has been reported as an alternative to fission. In this work, we also utilized this photoactivation mechanism. We confirmed that an electron LINAC as is typically used in nuclear medicine [17], can be used to produce 99Mo. However, it is not possible to use the current 99mTc generator to ensure adherence of Mo to the alumina column because a large amount of target Mo (stable isotope Mo) is contained in the mixture of 99Mo produced using the electron accelerator. Therefore, the generation of 99Mo by photoactivation does not carrier-free 99Mo.
The small adsorption capacity of alumina can be a significant concern, as the amount of absorbed Mo is typically in the range of 10 mg/g of alumina. For example, to produce 30 GBq of 99Mo via photonuclear reaction with 30 MeV photons and a 1 mA electron beam, 10 g of natural Mo should be irradiated for 10 h. Concerning the aforementioned rate, 1 kg of alumina would be needed for 10 g of the Mo target. Recent advances in the understanding of Mo adsorption to alumina are promising [18, 19], but the associated strategies have not been implemented thus far.
Equipment was developed to separate 99mTc via solvent extraction using methyl ethyl ketone [20]. This method uses natMoO3 as a target to separate 99mTc from 99Mo produced via 98Mo(n, γ)99Mo, 100Mo(p, d)99Mo, or 100Mo(γ, n)99Mo reactions using a two-tank separator. After 99mTc separation, it was reacted with MDP and applied to obtain a clear bone scintigram of a mouse [21].
Herein, we report a simple method for separating 99mTc from 99Mo/99mTc using Mo nanoparticles. The use of nanoparticles leads to the possibility of fixating the mother nuclide 99Mo mechanically instead of chemisorption, therefore circumventing the use of large amounts of alumina. However, under this conditions the daughter 99mTc can only be eluted from a column when 99Mo nuclei are recoiled out of the nanoparticles. Russian researchers have calculated the rate at which 99Mo recoils in the 100Mo(γ, n) 99Mo reaction using Mo nanoparticles that reach the particle surface has been calculated by Starovoitova et al. [22]. We conducted the first experiment to separate 99mTc from the recoiled 99Mo/99mTc using a method that is simpler than solvent extraction, which employs commercially available 99Mo/99mTc generator systems. Herein, Mo nanoparticles with diameters of several tens of nanometers and an alumina column were used.
Experimental
The production of 99Mo via nat.Mo(γ, n) 99Mo reaction was conducted at the electron LINAC facility at the Institute for Integrated Radiation and Nuclear Science, Kyoto University. Mo nanoparticles used therein possessed a commercially available natural isotope composition and the size was determined by x-ray diffraction. Metal Mo (100 mg) and MoO3 with the particle sizes of 35–45 and 13–80 nm, respectively, were used as powders or suspended in water. The samples used in the following experiments are presented in Table 1. Each target was sealed in a quartz tube and irradiated with bremsstrahlung γ-rays emitted from a 2-mm-thick Pt bombarded with 100 μA and 30 MeV electrons. A total of 2 MBq of 99Mo was obtained from these irradiations. After irradiation, the energy spectra of γ-rays from each target and the separated 99mTc solution were measured using a Ge detector. For the detector used for the gamma-ray measurement, energy calibration and detection efficiency were obtained in advance using standard sources. Furthermore, geometrical correction was performed by using Monte Carlo simulation.
As shown in Fig. 1, the irradiated Mo nanoparticles were added to a column filled with 1 cm3 of activated alumina in ion-exchanged water or saline. The generator vessel was made of polyethylene and the column filled with alumina had a diameter of 5 mm, a wall thickness of 1.5 mm and an overall height of 12 cm. For 99mTc separation, 2 mL of ion-exchanged water or saline was passed through the column. As shown in Fig. 1, the separated 99mTc solution was collected in a polyethylene tube and measured separately from the column with a Ge detector. The chemical separation and γ-ray measurement were repeated at regular intervals for one week, and the time dependence of radioactivity in the column and the radioactivity-separated 99mTc solution were measured. In addition, the nanoparticles were imaged using an electron microscope to investigate their size and shape.
Results and discussion
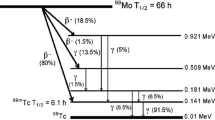
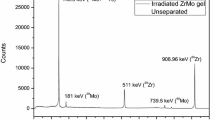
Figure 2 shows the γ-ray spectra of MoO3 after 3 h of irradiation and the effluent solution obtained by flowing ion-exchanged water through the MoO3 nanoparticles in the aluminum column. A 140.5 keV peak originated from 99mTc was observed. In addition, peaks were observed at 181.1, 366.4, 739.5, and 777.9 keV corresponding to 99Mo; the peak at 235.7 keV was attributed to 95mNb; 568.8 keV, 765.8 keV, 778.2 keV, 810.8 keV, and 1200.2 keV peaks from 96Nb. Figure 2b shows the γ-ray spectrum of the 99mTc solution separated from MoO3. Only the 140.5 keV peak from 99mTc was observed, indicating that all 99Mo, parent nuclei, and side reaction products of 95mNb and 96Nb were adsorbed onto the column. The percentage of radioisotopes remaining on the column was 99Mo > 99.5%, 95mNb > 97% and 96Nb > 98%, respectively.
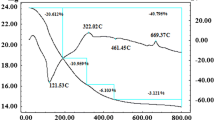
Figure 3 shows the decay curve of the 181.1 keV γ-ray in the effluent solution. A solid line was obtained through the least square fitting of the observed data. A linear fit was obtained through least square fitting of the observed data on a logarithmic scale, yielding a calculated half-life was 66.0 ± 0.6 h, being in good coincidence with the literature value 65.9 h. The radioactivity of 99mTc in the column before elution and in the eluate was determined at regular time intervals. Figure 4 shows the recovery rate of 99mTc from MoO3 as a function of time. In Fig. 4, the horizontal axis represents the elapsed time from the end of bombardment. The recovery was calculated at the time the radioactivity of 99mTc in the column was measured. The yield was defined as the ratio of 99mTc activity in the separated solution to that retained in the column. Data are shown for irradiation with powder alone, nanoparticles suspended in ion-exchanged water, and nanoparticles suspended in physiological saline. 99mTc yield was observed as a function of time for all samples. The recovery yields of 99mTc obtained in these experiments are listed in Table 2. The yields for Mo metal were significantly lower than those for MoO3, whereas those for MoO3 irradiated in aqueous conditions were higher than those under dry conditions.
Even while maintaining a constant MoO3 content, the separation yield of 99mTc for nanoparticles suspended in an aqueous solution was higher than that for the powdered form. This is because the individual particles diffused in the solvent and did not stick to each other during irradiation. However, if the particles were not first suspended in the solvent, they grew larger owing to gamma ray irradiation, and very few 99Mo/99mTc reached particle surface.
Considering the recovery rates of 99mTc in Mo and MO3 nanoparticles, the yield of MoO3 was approximately 20 times higher than that of Mo metal, primarily owing the nanoparticle shape. Figure 5 shows electron micrographs of the Mo and MoO3 nanoparticles. The Mo metal was spherical with a size of approximately 100 nm, while MoO3 was orthorhombic with a particle size of approximately 10 μm. While the recoil rate from the small size of particles is expected to increase owing to the large specific surface area, the results revealed that MoO3 with a large particle size exhibits a higher yield.
Dikiy et al. calculated the escape fraction of 99Mo produced via the 100Mo (γ, n) 99Mo reaction with photons possessing an end point energy of 30 MeV [23] and obtained values of 0.06 and 0.08 for 70 nm Mo and MoO3, respectively. The experimental results presented herein for 99mTc yield was 0.003 ± 0.001 for Mo, which is approximately 1/20 of the simulation results reported by Dikiny et al. This indicates that 99Mo recoiled from the inside of the nanoparticles to the surface. However, the probability of being trapped in the aqueous solution was low. For MoO3, the ratio of 99Mo trapped as 99mTc was 0.05 ± 0.006, yielding a trap efficiency of 0.6, which is significantly greater than that of Mo metal. Based on these experimental results, it seems that 99mTc can be separated with a higher yield by using even smaller MoO3 nanoparticles and performing gamma-ray irradiation in a state in which the particles do not stick together.
The separation of 99mTc was repeated several times at intervals of approximately one day. The yield increased for the MoO3 nanoparticles as a function of time, and the maximum separation yield of 99mTc was 14.4 ± 0.7% (Fig. 4). This indicated that the Mo nanoparticles acted as a 99mTc generator.
Conclusion
A novel 99mTc generator employing Mo nanoparticles and an alumina column was proposed. 99Mo was produced via a photonuclear reaction using naturally composed Mo metal and MoO3 nanoparticles as targets. The irradiated nanoparticles were poured into an alumina column, and 99mTc was separated at regular intervals using ion-exchanged water and saline. The nanoparticles suspended in the ion-exchanged water provided a higher 99mTc yield compared with the powdered nanoparticles. However, no difference in the 99mTc yield was observed between ion-exchanged water and saline. The maximum separation 99mTc yield was 14.4 ± 0.7%.
References
Delaloye B, Delaloye-Bischof A, Dudczak R, Koppenhagen K, Mata F, Penafiel A, Maul FD, Pasquier J (1985) Clinical comparison of 99mTc-HMDP and 99mTc-MDP. Eur J Nucl Med 11:182–185
Alam MS, Takeuchi R, Kasagi K, Misaki T, Miyamoto S, Iida Y, Hidaka A, Konishi N (1997) Value of combined technetium-99m hydroxy methylene diphosphonate and thallium-201 imaging in detecting bone metastases from thyroid carcinoma. Thyroid 7:705–712
Japan Radioisotope Association (2020) Statics on the distribution of radioisotopes in Japan 2020 (in Japanese). Japan Radioisotope Association Tokyo
Mabuchi N, Hamada T, Ishikawa K, Sakashita T, Shibata Y et al (1983) Clinical comparison of 99mTc diethylenetriamine pentaacetic acid-human serum albumin (99mTc-HAS-D) and 99mTc-human serum albumin (99mTc-HAS) for cardiac blood pool imaging. Radioisotopes 37(7):380–386
Gandric S, Sonik B, Subramanyam P, Palanisway S (2013) 99mTc-MAA scintigraphy-indications other than pulmonary embolism-a pictorial essay. J Nucl Med 54:1263–1269
Committee on State of Molybdenum-99 Production and utilization and progress toward eliminating use of highly enriched uranium nuclear and radiation studies board division on earth and life studies (2016) Molybdenum-99/Technetium-99m supply National Academies of Sciencies・Engineering・Medicine 187-200 2016
Fujita Y, Seki M, Sano T, Fujihara Y, Kitagawa T et al (2021) Effect on 99Mo-adsorption/99mTc-elution properties of alumina with different surface structures. J Radioanal Nucl Chem 327:1355–1363
IAEA-TECDOC-1065 (1999) Production technologies of molybdenum-99 and technetium-99m IEEA Vienna
htp:// JSNM.org./archives/2827/ Technetium product supply Q & A Japanese Society of Nuclear Medicine
Ruth T (2020) The shortage of Tc-99m. Annu Rev Nucl Part Sci 20:77–94
IAEA radioisotopes and radiopharmaceutical reports (2019) Cyclotron based production of technetium-99m IAEA Vienna
IAEA (1999)-TECDOC-1066 Production technologies for molybdenum-99 and technetium-99m IAEA Vienna
Nagai Y, an Hatsukawa Y, (2009) Production of 99Mo for nuclear medicine by 100Mo(n,2n)99Mo. J Phys Soc JP 78:033201–033204
Nakai K, Takahashi N, Hatazawa J, Shinohara A, Hayashi Y, Ikeda H, Kanai Y, Watabe T, Fukuda M, Hatanaka K (2014) Feasibility studies towards future self-sufficient supply of the 99Mo-99mTc isotopes with Japanese accelerators. Proc Jpn Acad B90:413–421
Kikunaga H, (2015) presentation/part2/Med Sci Kickoff Sympo RI production via photonuclear reaction with electron linear accelerator (in Japanese) http://www.rcnp.osaka-u.ac.jp/MedSciSympo2015Kikunagapdf
Inagaki M, Sekimoto S, Tadokoro T, Ueno Y, Kani Y, Ohtsuki T (2020) Production of 99Mo/99mTc by photonuclear reaction using a natMoO3 target. J Radioanal Nucl Chem 324:681–686
Takeda T, Fujiwara M, Kurosawa M, Takahashi N, Tamura M, Fujikawa Y, Suzuki N, Abe N, Kubota T, Takahashi T (2018) 99mTc production via the (γ, n) reaction on natural Mo. J Radional Nucl Chem 318:811–821
Tanase M, Tatenuma K, Ishikawa K, Kurosawa K, Nishino M, Hasegawa Y (1997) A 99mTc generator using a new inorganic polymer adsorbent for (n, γ)99Mo. Appl Radiat Isot 48:607–611
IAEA (1995) Alternative technologies for 99Tcm generators. TECDOC-852 4:11–15
Takahashi N (2020) Technetiun-99m separation system and technetium-99m separation method. International patent PCT/JP2020/007936
Takahashi N, Nakai K, Shinohara A, Hatazawa J, Nakamura M, Fukuda M, Hatanaka K, Morikawa Y, Kobayashi M, Yamamoto (2012) Production of 99Mo-99mTc by using spallation neutron. 2012 SNM annual meeting Miami
Starovoitova VN, Tchelidze L, Wells DP (2014) Production of medical radioisotopes with linear accelerators. Appl Radiat Isot 85:39–44
Dikiy NP, Dovbnya AN, Fedorchenko DV, Khazhmuradov MA (2016) GEANT 4 simulation of 99Mo photonuclear production in nanoparticles. Appl Radiat Isot 114:7–13
Acknowledgements
This study was performed at the electron LINAC facility at the Institute for Integrated Radiation and Nuclear Science, Kyoto University under a user program (Proposal No. R3081). We would like to thank Editage (www.editage.com) for English language editing.
Funding
Open access funding provided by Osaka University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Consent for publication
All data generated or analyzed during this study are included in this published article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takahashi, N., Fujiwara, M., Kurosawa, M. et al. 99mTc generator using molybdenum nanoparticles. J Radioanal Nucl Chem 333, 17–22 (2024). https://doi.org/10.1007/s10967-023-09173-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09173-z