Abstract
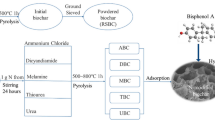
Biomass transformation is considered as a green, economical and sustainable strategy in the field of wastewater treatment. Herein, we demonstrate a novel amino-reinforced phosphorylated biochar by the modified phosphoric acid (H3PO4)/urea/N,N-dimethylformamide method for highly effective U(VI) capture. The introduction of urea provides new amino sites on the surface of carbon and plays a decisive role in the phosphorylation modification of biochar. The adsorption process of the amino-reinforced phosphorylated biochar presents pseudo-second-order kinetics, and the maximum adsorption capacity calculated by Langmuir isotherm model can reach 150.38 mg g−1. Moreover, the adsorbent still maintains an excellent removal efficiency after four adsorption–desorption recycles and shows superior selectivity. Structural analysis results after adsorption demonstrate that the adsorption performance of phosphorylated carbon is predominantly attributed to the chelating and electrostatic interaction.












Similar content being viewed by others
References
Zhang C, Cui W, Niu C, Yi S, Liang R, Qi J, Chen X, Jiang W, Zhang L, Qiu J (2021) RGO-based covalent organic framework hydrogel for synergistically enhance uranium capture capacity through photothermal desalination. Chem Eng J 428:131178. https://doi.org/10.1016/j.cej.2021.131178
Zhao S, Feng T, Feng L, Yan B, Sun W, Luo G, Wang M, Jian Y, Liu T, Yuan Y, Wang N (2022) Rapid recovery of uranium with magnetic-single-molecular amidoxime adsorbent. Sep Purif Technol 287:120524. https://doi.org/10.1016/j.seppur.2022.120524
Xu M, Zhou L, Zhang L, Zhang S, Chen F, Zhou R, Hua D (2022) Two-dimensional imprinting strategy to create specific nanotrap for selective uranium adsorption with ultrahigh capacity. ACS Appl Mater Interfaces 14(7):9408–9417. https://doi.org/10.1021/acsami.1c20543
Xiong T, Li Q, Liao J, Zhang Y, Zhu W (2022) Highly enhanced adsorption performance to uranium (VI) by facile synthesized hydroxyapatite aerogel. J Hazard Mater 423:127184. https://doi.org/10.1016/j.jhazmat.2021.127184
Ji Y, Xu F, Wei W, Gao H, Zhang K, Zhang G, Xu Y, Zhang P (2021) Efficient and fast adsorption of methylene blue dye onto a nanosheet MFI zeolite. J Solid State Chem 295:121917. https://doi.org/10.1016/j.jssc.2020.121917
Liu T, Zhang X, Gu A, Liu Y, Chen M, Wang H, Zhang R, Tang S, Xie Z, Wang N (2022) In-situ grown bilayer MOF from robust wood aerogel with aligned microchannel arrays toward selective extraction of uranium from seawater. Chem Eng J 433:134346. https://doi.org/10.1016/j.cej.2021.134346
Wang Y, Lin K, Liu Y, Deng X (2022) Nanocomposites of functionalized metal organic frameworks and magnetic graphene oxide for selective adsorption and efficient determination of Lead(II). J Solid State Chem 313:123300. https://doi.org/10.1016/j.jssc.2022.123300
Zhao W, Lin X, Cai H, Mu T, Luo X (2017) Preparation of mesoporous carbon from sodium lignosulfonate by hydrothermal and template method and its adsorption of uranium (VI). Ind Eng Chem Res 56(44):12745–12754. https://doi.org/10.1021/acs.iecr.7b02854
Jiang D, Li M, Wang Y (2016) Biochar synthesis and applications in energy storage and conversion. Green Chem 18:4824–4854. https://doi.org/10.1039/c6gc01172a
Wang J, Nie P, Ding B, Dong S, Hao X, Dou H, Zhang X (2017) Biomass derived carbon for energy storage devices. J Mater Chem A 5(6):2411–2428. https://doi.org/10.1039/c6ta08742f
Guilhen SN, Rovani S, Araujo LG, Tenorio JAS, Masek O (2021) Uranium removal from aqueous solution using macauba endocarp-derived biochar: effect of physical activation. Environ Pollut 272:116022. https://doi.org/10.1016/j.envpol.2020.116022
Turbak AF, Snyder FW, Sandberg KR (1983) Microfibrillated cellulose, a new cellulose product properties, uses, and commercial potential. J Appl Polym Sci 37:815–827
Li T, Chen C, Brozena A, Zhu J, Xu L, Driemeier C, Dai J, Rojas OJ, Isogai A, Wagberg L, Hu L (2021) Developing fibrillated cellulose as a sustainable technological material. Nature 590(7844):47–56. https://doi.org/10.1038/s41586-020-03167-7
Yuan L, Liu Y, Shi W, Lv Y, Lan J, Zhao Y, Chai Z (2011) High performance of phosphonate-functionalized mesoporous silica for U(VI) sorption from aqueous solution. Dalton Trans 40(28):7446–7453. https://doi.org/10.1039/c1dt10085h
Yue Y, Sun X, Mayes R, Kim J, Fulvio PF, Qiao Z, Brown S, Tsouris C, Oyola Y, Dai S (2013) Polymer-coated nanoporous carbons for trace seawater uranium adsorption. Sci China Chem 56(11):1510–1515. https://doi.org/10.1007/s11426-013-4995-5
Yang P, Liu Q, Liu J, Chen R, Li R, Bai X, Wang J (2019) Highly efficient immobilization of uranium(VI) from aqueous solution by phosphonate-functionalized dendritic fibrous nanosilica (DFNS). J Hazard Mater 363:248–257. https://doi.org/10.1016/j.jhazmat.2018.09.062
Lehtonen J, Hassinen J, Kumar AA, Johansson LS, Mäenpää R, Pahimanolis N, Pradeep T, Ikkala O, Rojas OJ (2020) Phosphorylated cellulose nanofibers exhibit exceptional capacity for uranium capture. Cellulose 27(18):10719–10732. https://doi.org/10.1007/s10570-020-02971-8
Chen H, Wang Y, Zhao W, Xiong G, Cao X, Dai Y, Le Z, Zhang Z, Liu Y (2017) Phosphorylation of graphehe oxide to improve adsorption of U(VI) from aquaeous solutions. J Radioanal Nucl Chem 313(1):175–189. https://doi.org/10.1007/s10967-017-5274-2
Zhou L, Huang Z, Luo T, Jia Y, Liu Z, Adesina AA (2014) Biosorption of uranium(VI) from aqueous solution using phosphate-modified pine wood sawdust. J Radioanal Nucl Chem 303:1917–1925. https://doi.org/10.1007/s10967-014-3725-6
Shukla JP, Misra SK (1991) Carrier-mediated transport of uranyl ions across tributyl phosphate-dodecane liquid membranes. J Membr Sci 64(1):93–102. https://doi.org/10.1016/0376-7388(91)80080-P
Deng S, Yu C, Niu J, Liao J, Liu X (2020) Microwave assisted synthesis of phosphorylated PAN fiber for highly efficient and enhanced extraction of U(VI) ions from water. Chem Eng J 392:123815. https://doi.org/10.1016/j.cej.2019.123815
Yu J, Yuan L, Wang S (2019) Phosphonate-decorated covalent organic frameworks for actinide extraction: a breakthrough under highly acidic conditions. CCS Chem 3:286–295. https://doi.org/10.31635/ccschem.019.20190005
Zhang N, Li J, Tian B (2023) Phosphorylated cellulose carbamate for highly effective capture of U(VI). J Radioanal Nucl Ch 332:173–183. https://doi.org/10.1007/s10967-022-08678-3
Guilhen SN, Rovani S, Filho LP, Fungaro DA (2018) Kinetic study of uranium removal from aqueous solutions by macaúba biochar. Chem Eng Commun 206(11):1354–1366. https://doi.org/10.1080/00986445.2018.1533467
Fu F, Xu M, Wang H, Wang Y, Ge H, Zhou J (2015) Improved synthesis of cellulose carbamates with minimum urea based on an easy scale-up method. ACS Sustain Chem Eng 3(7):1510–1517. https://doi.org/10.1021/acssuschemeng.5b00219
Duan S, Ma W, Pan Y, Meng F, Yu S, Wu L (2017) Synthesis of magnetic biochar from iron sludge for the enhancement of Cr (VI) removal from solution. J Taiwan Inst Chem Eng 80:835–841. https://doi.org/10.1016/j.jtice.2017.07.002
Shen F, Hu Y, Guan P, Ren X (2012) Ti(4+)-phosphate functionalized cellulose for phosphopeptides enrichment and its application in rice phosphoproteome analysis. J Chromatogr B 902:108–115. https://doi.org/10.1016/j.jchromb.2012.06.033
Guilhen SN, Mašek O, Ortiz N, Izidoro JC, Fungaro DA (2019) Pyrolytic temperature evaluation of macauba biochar for uranium adsorption from aqueous solutions. Biomass Bioenergy 122:381–390. https://doi.org/10.1016/j.biombioe.2019.01.008
Wang L, Dong X, Jiang H, Li ZM (2014) Phosphorylated ordered mesoporous carbon as a novel solid acid catalyst for the esterification of oleic acid. Catal Commun 56:164–167. https://doi.org/10.1016/j.catcom.2014.07.008
Zhang Z, Dong Z, Wang X, Dai Y, Cao X, Wang Y, Hua R, Feng H, Chen J, Liu Y, Hu B, Wang X (2019) Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U(VI): batch and fixed-bed column studies. Chem Eng J 370:1376–1387. https://doi.org/10.1016/j.cej.2019.04.012
Dai Y, Zhou L, Tang X, Xi J, Ouyang J, Liu Z, Huang G, Adesina A (2020) Macroporous ion-imprinted chitosan foams for the selective biosorption of U(VI) from aqueous solution. Int J Biol Macromol 164:4155–4164. https://doi.org/10.1016/j.ijbiomac.2020.08.238
Zhao H, Qi C, Yan X, Ji J, Chai Z, Wang S, Zheng T (2022) A multifunctional porous uranyl phosphonate framework for cyclic utilization: salvages, uranyl leaking prevention, and fluorescent sensing. Chem Eng J 429:132474. https://doi.org/10.1016/j.cej.2021.132474
Zhang G, Fang Y, Wang Y, Liu L, Mei D, Ma F, Meng Y, Dong H, Zhang C (2022) Synthesis of amino acid modified MIL-101 and efficient uranium adsorption from water. J Mol Liq 349:118095. https://doi.org/10.1016/j.molliq.2021.118095
Zhang G, Wang Y, Zhang X, Liu L, Ma F, Zhang C, Dong H (2022) Synthesis of a porous amidoxime modified hypercrosslinked benzil polymer and efficient uranium extraction from water. Colloid Surf A 641:128508. https://doi.org/10.1016/j.colsurfa.2022.128508
Cai Y, Chen L, Yang S, Xu L, Qin H, Liu Z, Chen L, Wang X, Wang S (2019) Rational synthesis of novel phosphorylated chitosan-carboxymethyl cellulose composite for highly effective decontamination of U(VI). Acs Sustain Chem Eng 7(5):5393–5403. https://doi.org/10.1021/acssuschemeng.8b06416
Zhang Z, Li Z, Dong Z, Yu F, Wang Y, Wang Y, Cao X, Liu Y, Liu Y (2022) Synergy of photocatalytic reduction and adsorption for boosting uranium removal with PMo12/UiO-66 heterojunction. Chin Chem Lett 33(7):3577–3580. https://doi.org/10.1016/j.cclet.2022.01.062
Liu Y, Wang Y, Xia H et al (2022) Low-cost reed straw-derived biochar prepared by hydrothermal carbonization for the removal of uranium(VI) from aqueous solution. J Radioanal Nucl Chem 331:3915–3925. https://doi.org/10.1007/s10967-022-08421-y
Albayari M, Nazal MK, Khalili FI, Nordin N, Adnan R (2021) Biochar derived from Salvadora persica branches biomass as low-cost adsorbent for removal of uranium(VI) and thorium(IV) from water. J Radioanal Nucl Chem 328:669–678. https://doi.org/10.1007/s10967-018-6358-3
Xu Z, Xing Y, Ren A, Ma D, Li Y, Hu S (2020) Study on adsorption properties of water hyacinth-derived biochar for uranium (VI). J Radioanal Nucl Chem 324:1317–1327. https://doi.org/10.1007/s10967-020-07160-2
Li L, Yang M, Lu Q, Zhu W, Ma H, Dai L (2019) Oxygen-rich biochar from torrefaction: a versatile adsorbent for water pollution control. Bioresour Technol 294:122142. https://doi.org/10.1016/j.biortech.2019.122142
Li N, Yin M, Tsang DCW, Yang S, Liu J, Li X, Song G, Wang J (2019) Mechanisms of U(VI) removal by biochar derived from Ficus microcarpa aerial root: a comparison between raw and modified biochar. Sci Total Environ 697:134115. https://doi.org/10.1016/j.scitotenv.2019.134115
Zhou Y, Xiao J, Hu R, Wang T, Shao X, Chen G, Chen L, Tian X (2020) Engineered phosphorous-functionalized biochar with enhanced porosity using phytic acid-assisted ball milling for efficient and selective uptake of aquatic uranium. J Mol Liq 303:112659. https://doi.org/10.1016/j.molliq.2020.112659
Ahmed W, Mehmood S, Qaswar M, Ali S, Khan Z, Ying H, Chen D, Núñez-Delgado A (2021) Oxidized biochar obtained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J Environ Chem Eng 9(2):105104. https://doi.org/10.1016/j.jece.2021.105104
Acknowledgements
This work was supported by the Science Research Foundation of Heilongjiang Academy of Sciences [KY2022YZN01], Scientific Research Business Fund Project of Heilongjiang Provincial Research Institutes [CZKYF2022-1-C011], Sciences Talent Team Construction Platform Project of Heilongjiang Academy of Sciences [RC2022YZN01], Special Project of Heilongjiang Academy of Sciences [YZQY2023YZNY01], Provincial-level ecological and environmental protection scientific research project [HST2022H003], Dean Fund of Heilongjiang Provincial Academy of Sciences [YZ2023YZNY02], the Natural Science Foundation of Heilongjiang Province (LH2023A021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, N., Li, J., Tian, B. et al. The preparation of amino-reinforced phosphorylated biochar for efficient uranium adsorption. J Radioanal Nucl Chem 332, 3305–3315 (2023). https://doi.org/10.1007/s10967-023-09025-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09025-w