Abstract
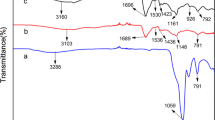
To efficiently remove U(VI) from aqueous solutions, porous polyvinyl alcohol/carboxylated carbon nanotubes/sodium alginate (PPCS) hydrogel microspheres were prepared using the physical cross-linking method. The successful formation of highly porous PPCS hydrogel microspheres was confirmed by SEM and BET. Moreover, PPCS hydrogel microspheres were recovered from aqueous solutions more easily than carbon nanotubes. The main mechanism of PPCS for U(VI) removal may be the chelation of hydroxyl and carboxyl groups. PPCS may be a promising and recyclable adsorbent for uranium extraction.
Graphical abstract







Similar content being viewed by others

References
Rahman ROA, Ibrahium HA, Hung Y-T (2011) Liquid radioactive wastes treatment: a review. Water 3:551–565. https://doi.org/10.3390/w3020551
Wang Q, Gao Q, Al-Enizi AM, Nafady A, Ma S (2020) Recent advances in MOF-based photocatalysis: environmental remediation under visible light. Inorg Chem Front 7:300–339. https://doi.org/10.1039/c9qi01120j
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331. https://doi.org/10.1016/j.jhazmat.2011.10.016
Bagda E, Tuzen M, Sari A (2017) Equilibrium, thermodynamic and kinetic investigations for biosorption of uranium with green algae (Cladophora hutchinsiae). J Environ Radioact 175:7–14. https://doi.org/10.1016/j.jenvrad.2017.04.004
Saleh TA, Naeemullah TM, Sarı A (2017) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227. https://doi.org/10.1016/j.cherd.2016.10.030
Deb AKS, Ilaiyaraja P, Ponraju D, Venkatraman B (2011) Diglycolamide functionalized multi-walled carbon nanotubes for removal of uranium from aqueous solution by adsorption. J Radioanal Nucl Chem 291:877–883. https://doi.org/10.1007/s10967-011-1366-6
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462. https://doi.org/10.1021/es203439v
Perreault F, Fonseca de Faria A, Elimelech M (2015) Environmental applications of graphene-based nanomaterials. Chem Soc Rev 44:5861–5896. https://doi.org/10.1039/c5cs00021a
Liu X, Wang M, Zhang S, Pan B (2013) Application potential of carbon nanotubes in water treatment: a review. J Environ Sci 25:1263–1280. https://doi.org/10.1016/s1001-0742(12)60161-2
Tian Y, Gao B, Morales VL, Wu L, Wang Y, Muñoz-Carpena R, Cao C, Huang Q, Yang L (2012) Methods of using carbon nanotubes as filter media to remove aqueous heavy metals. Chem Eng J 210:557–563. https://doi.org/10.1016/j.cej.2012.09.015
Gupta VK, Moradi O, Tyagi I, Agarwal S, Sadegh H, Shahryari-Ghoshekandi R, Makhlouf ASH, Goodarzi M, Garshasbi A (2016) Study on the removal of heavy metal ions from industry waste by carbon nanotubes: effect of the surface modification: a review. Crit Rev Environ Sci Technol 46:93–118. https://doi.org/10.1080/10643389.2015.1061874
Stafiej A, Pyrzynska K (2007) Adsorption of heavy metal ions with carbon nanotubes. Sep Purif Technol 58:49–52
Zhang S, Han D, Ding Z, Wang X, Zhao D, Hu Y, Xiao X (2019) Fabrication and characterization of one interpenetrating network hydrogel based on sodium alginate and polyvinyl alcohol. J Wuhan Univ Technol-Mater Sci Ed 34:744–751. https://doi.org/10.1007/s11595-019-2112-0
Guo Y, Liu XY, Xie S, Liu H, Wang C, Wang L (2022) 3D ZnO modified biochar-based hydrogels for removing U(VI) in aqueous solution. Colloids Surf A Physicochem Eng Asp 642:128606. https://doi.org/10.1016/j.colsurfa.2022.128606
Liu XY, Xie SB, Wang GH, Huang X, Duan Y, Liu HY (2021) Fabrication of environmentally sensitive amidoxime hydrogel for extraction of uranium (VI) from an aqueous solution. Colloids Surf A Physicochem Eng Asp 611:125813. https://doi.org/10.1016/j.colsurfa.2020.125813
Gao X, Guo C, Hao J, Zhao Z, Long H, Li M (2020) Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int J Biol Macromol 164:4423–4434. https://doi.org/10.1016/j.ijbiomac.2020.09.046
Inal M, Erduran N (2015) Removal of various anionic dyes using sodium alginate/poly(N-vinyl-2-pyrrolidone) blend hydrogel beads. Polym Bull 72:1735–1752. https://doi.org/10.1007/s00289-015-1367-7
Long J, Wang Y, Xu Y, Li X (2015) An innovative approach for separation and purification of natural products using carbon nanotube-alginate gel beads as a novel stationary phase. RSC Adv 5:10878–10885. https://doi.org/10.1039/c4ra12732c
Khosroshahi ME, Ghazanfari L (2012) Preparation and rheological studies of uncoated and PVA-coated magnetite nanofluid. J Magn Magn Mater 324:4143–4146. https://doi.org/10.1016/j.jmmm.2012.07.025
Sauerwein M, Steeb H (2020) Modeling of dynamic hydrogel swelling within the pore space of a porous medium. Int J Eng Sci 155:103353. https://doi.org/10.1016/j.ijengsci.2020.103353
Trikkaliotis DG, Christoforidis AK, Mitropoulos AC, Kyzas GZ (2020) Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr Polym 234:115890. https://doi.org/10.1016/j.carbpol.2020.115890
Algothmi WM, Bandaru NM, Yu Y, Shapter JG, Ellis AV (2013) Alginate-graphene oxide hybrid gel beads: an efficient copper adsorbent material. J Colloid Interface Sci 397:32–38. https://doi.org/10.1016/j.jcis.2013.01.051
Di Donato P, Taurisano V, Poli A, d’Ayala GG, Nicolaus B, Malinconinco M, Santagata G (2020) Vegetable wastes derived polysaccharides as natural eco-friendly plasticizers of sodium alginate. Carbohydr Polym 229:115427. https://doi.org/10.1016/j.carbpol.2019.115427
Ilkhanizadeh S, Khalafy J, Dekamin MG (2019) Sodium alginate: a biopolymeric catalyst for the synthesis of novel and known polysubstituted pyrano 3,2-c chromenes. Int J Biol Macromol 140:605–613. https://doi.org/10.1016/j.ijbiomac.2019.08.154
Fan J, Shi Z, Lian M, Li H, Yin J (2013) Mechanically strong graphene oxide/sodium alginate/polyacrylamide nanocomposite hydrogel with improved dye adsorption capacity. J Mater Chem A. https://doi.org/10.1039/c3ta10639j
Siddiqui MN, Redhwi HH, Tsagkalias I, Softas C, Ioannidou MD, Achilias DS (2016) Synthesis and characterization of poly(2-hydroxyethyl methacrylate)/silver hydrogel nanocomposites prepared via in situ radical polymerization. Thermochim Acta 643:53–64. https://doi.org/10.1016/j.tca.2016.09.017
Shehzad H, Zhou L, Wang Y, Ouyang J, Huang G, Liu Z, Li Z (2019) Effective biosorption of U(VI) from aqueous solution using calcium alginate hydrogel beads grafted with amino-carbamate moieties. J Radioanal Nucl Chem 321:605–615. https://doi.org/10.1007/s10967-019-06631-5
Yi X, Sun F, Han Z, Han F, He J, Ou M, Gu J, Xu X (2018) Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu (II) and U (VI) removal. Ecotoxicol Environ Saf 158:309–318. https://doi.org/10.1016/j.ecoenv.2018.04.039
Kong L, Ruan Y, Zheng Q, Su M, Diao Z, Chen D, La H, Chang X, Shih K (2020) Uranium extraction using hydroxyapatite recovered from phosphorus containing wastewater. J Hazard Mater 382:120784. https://doi.org/10.1016/j.jhazmat.2019.120784
Sun Y, Ding C, Cheng W, Wang X (2014) Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J Hazard Mater 280:399–408. https://doi.org/10.1016/j.jhazmat.2014.08.023
Tripathi A, Melo JS (2019) Self-assembled biogenic melanin modulated surface chemistry of biopolymers-colloidal silica composite porous matrix for the recovery of uranium. J Appl Polym Sci 136:46937. https://doi.org/10.1002/app.46937
Wang X, Yu S, Wu Y, Pang H, Yu S, Chen Z, Hou J, Alsaedi A, Hayat T, Wang S (2018) The synergistic elimination of uranium (VI) species from aqueous solution using bi-functional nanocomposite of carbon sphere and layered double hydroxide. Chem Eng J 342:321–330. https://doi.org/10.1016/j.cej.2018.02.102
Tang X, Zhou L, Le Z, Wang Y, Liu Z, Huang G, Adesina AA (2020) Preparation of porous chitosan/carboxylated carbon nanotube composite aerogels for the efficient removal of uranium(VI) from aqueous solution. Int J Biol Macromol 160:1000–1008. https://doi.org/10.1016/j.ijbiomac.2020.05.179
Han B, Zhang E, Cheng G, Zhang L, Wang D, Wang X (2018) Hydrothermal carbon superstructures enriched with carboxyl groups for highly efficient uranium removal. Chem Eng J 338:734–744. https://doi.org/10.1016/j.cej.2018.01.089
Zhao F, Repo E, Song Y, Yin D, Ben Hammouda S, Chen L, Kalliola S, Tang J, Tam KC, Sillanpaa M (2017) Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study. Green Chem 19:4816–4828. https://doi.org/10.1039/c7gc01770g
Liao J, Zhang Y (2020) Effective removal of uranium from aqueous solution by using novel sustainable porous Al2O3 materials derived from different precursors of aluminum. Inorg Chem Front 7:765–776. https://doi.org/10.1039/c9qi01426h
Wei J, Zhang W, Pan W, Li C, Sun W (2018) Experimental and theoretical investigations on Se(iv) and Se(vi) adsorption to UiO-66-based metal-organic frameworks. Environ Sci-Nano 5:1441–1453. https://doi.org/10.1039/c8en00180d
Liu L, Lin X, Li M, Chu H, Wang H, Xie Y, Du Z, Liu M, Liang L, Gong H, Zhou J, Li Z, Luo X (2021) Microwave-assisted hydrothermal synthesis of carbon doped with phosphorus for uranium(VI) adsorption. J Radioanal Nucl Chem 327:73–89. https://doi.org/10.1007/s10967-020-07453-6
Shao D, Jiang Z, Wang X, Li J, Meng Y (2009) Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO22+ from aqueous solution. J Phys Chem B 113:860–864. https://doi.org/10.1021/jp8091094
Allaboun H, Fares M, Abu Al-Rub F (2016) Removal of uranium and associated contaminants from aqueous solutions using functional carbon nanotubes-sodium alginate conjugates. Minerals 6:9. https://doi.org/10.3390/min6010009
Zong P, Wang S, Zhao Y, Wang H, Pan H, He C (2013) Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions. Chem Eng J 220:45–52. https://doi.org/10.1016/j.cej.2013.01.038
Kim JH, Lee HI, Yeon J-W, Jung Y, Kim JM (2010) Removal of uranium(VI) from aqueous solutions by nanoporous carbon and its chelating polymer composite. J Radioanal Nucl Chem 286:129–133. https://doi.org/10.1007/s10967-010-0624-3
Zhao G, Wen T, Yang X, Yang S, Liao J, Hu J, Shao D, Wang X (2012) Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalton Trans 41:6182–6188. https://doi.org/10.1039/c2dt00054g
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@SiO2 core-shell magnetic microspheres for highly efficient sorption of U(VI). Chem Eng J 235:275–283. https://doi.org/10.1016/j.cej.2013.09.034
Ouyang J, Wang Y, Li T, Zhou L, Liu Z (2018) Immobilization of carboxyl-modified multiwalled carbon nanotubes in chitosan-based composite membranes for U(VI) sorption. J Radioanal Nucl Chem 317:1419–1428. https://doi.org/10.1007/s10967-018-5993-z
Chen Y, Ning P, Miao R, He L, Guan Q (2021) Resource utilization of agricultural residues: one-step preparation of biochar derived from Pennisetum giganteum for efficiently removing chromium from water in a wide pH range. Environ Sci Pollut Res 28:69381–69392. https://doi.org/10.1007/s11356-021-15388-y
Wang Y, Wang Z, Ang R, Yang J, Liu N, Liao J, Yang Y, Tang J (2015) Synthesis of amidoximated graphene oxide nanoribbons from unzipping of multiwalled carbon nanotubes for selective separation of uranium(vi). RSC Adv 5:89309–89318. https://doi.org/10.1039/c5ra15977f
Wang Z, Kang HJ, Zhang W, Zhang SF, Li JZ (2017) Improvement of interfacial interactions using natural polyphenol-inspired tannic acid-coated nanoclay enhancement of soy protein isolate biofilms. Appl Surf Sci 401:271–282. https://doi.org/10.1016/j.apsusc.2017.01.015
Song W, Wang X, Wang Q, Shao D, Wang X (2015) Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys Chem Chem Phys 17:398–406. https://doi.org/10.1039/c4cp04289a
Liu S, Li J, Xu S, Wang M, Zhang Y, Xue X (2019) A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour Technol 282:48–55. https://doi.org/10.1016/j.biortech.2019.02.092
Zhang X, Lin X, He Y, Luo X (2019) Phenolic hydroxyl derived copper alginate microspheres as superior adsorbent for effective adsorption of tetracycline. Int J Biol Macromol 136:445–459. https://doi.org/10.1016/j.ijbiomac.2019.05.165
Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province (No.2020JJ30579)
Author information
Authors and Affiliations
Contributions
YJ: Data curation, Writing—original draft. SX: Project administration, Supervision. YD, GW: Revising paper. YG, CW: Reviewing paper and English language polishing.
Corresponding author
Ethics declarations
Conflict of interest
There were no known conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jian, Y., Xie, S., Duan, Y. et al. Porous carboxylated carbon nanotubes hydrogel microspheres for removing U(VI) from aqueous solutions. J Radioanal Nucl Chem 332, 2679–2689 (2023). https://doi.org/10.1007/s10967-023-08916-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08916-2