Abstract
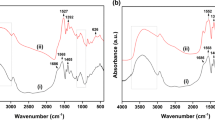
Polyacrylic acid, Chitosan and nanosilica particles composite (PCNS) was prepared for enrichment of U (VI) from aqueous solutions. Adsorption tests controlled by different parameters including contact time, pH, initial concentration of UO22+ and coexistence ions were examined. FTIR, SEM and EDX studies proved the formation of composite and confirmed efficient adsorption of UO22+ by PCNS. The experimental datas fit the Langmuir and pseudo-second-order models, the RL (0.115–0.645) indicates the adsorption of UO22+ onto PCNS are favorable. The value of qm (451.118 mg g−1) and adsorption–desorption experiments showed PCNS hydrogel can be reckoned as a high efficienct and sustainable material for removal of U (VI).











Similar content being viewed by others
References
Xie S, Yang J, Chen C, Zhang X, Wang Q, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freundii. J Environ Radioact 99(1):126–133. https://doi.org/10.1016/j.jenvrad.2007.07.003
Yuan L, Sun M, Liao X, Zhao Y, Chai Z, Shi W (2014) Solvent extraction of U (VI) by trioctylphosphine oxide using a room-temperature ionic liquid. Sci China Chem 57(11):1432–1438. https://doi.org/10.1007/s11426-014-5194-8
Akar ST, Akar T, Kaynak Z, Anilan B, Cabuk A, Tabak Ö, Demir TA, Gedikbey T (2009) Removal of copper(II) ions from synthetic solution and real wastewater by the combined action of dried Trametes versicolor cells and montmorillonite. Hydrometallurgy 97(1–2):98–104. https://doi.org/10.1016/j.hydromet.2009.01.009
Akar T, Tunali S (2006) Biosorption characteristics of Aspergillus flavus biomass for removal of Pb(II) and Cu(II) ions from an aqueous solution. Bioresour Technol 97(15):1780–1787. https://doi.org/10.1016/j.biortech.2005.09.009
Han R, Zhang J, Zou W, Xiao H, Shi J, Liu H (2006) Biosorption of copper(II) and lead(II) from aqueous solution by chaff in a fixed-bed column. J Hazard Mater 133(1–3):262–268. https://doi.org/10.1016/j.jhazmat.2005.10.019
Al-Rub FAA, El-Naas MH, Ashour I, Al-Marzouqi M (2006) Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process Biochem 41(2):457–464. https://doi.org/10.1016/j.procbio.2005.07.018
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Yi X, Xu Z, Liu Y, Guo X, Ou M, Xu X (2017) Highly efficient removal of uranium(vi) from wastewater by polyacrylic acid hydrogels. RSC Adv 7(11):6278–6287. https://doi.org/10.1039/c6ra26846c
Abou El-Reash YG, Abdelghany AM, Elrazak AA (2016) Removal and separation of Cu(II) from aqueous solutions using nano-silver chitosan/polyacrylamide membranes. Int J Biol Macromol 86:789–798. https://doi.org/10.1016/j.ijbiomac.2016.01.101
Vakili M, Rafatullah M, Salamatinia B, Abdullah AZ, Ibrahim MH, Tan KB, Gholami Z, Amouzgar P (2014) Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review. Carbohydr Polym 113:115–130. https://doi.org/10.1016/j.carbpol.2014.07.007
Jin Li JC (2013) Parametric optimization of extracellular chitin deacetylase production by scopulariopsis brevicaulis. J Biocatal Biotransform. https://doi.org/10.4172/2324-9099.1000103
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(7):603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Dotto GL, Pinto LAA (2011) Adsorption of food dyes onto chitosan: optimization process and kinetic. Carbohydr Polym 84(1):231–238. https://doi.org/10.1016/j.carbpol.2010.11.028
Shehzad H, Zhou L, Li Z, Chen Q, Wang Y, Liu Z, Adesina AA (2017) Effective adsorption of U (VI) from aqueous solution using magnetic chitosan nanoparticles grafted with maleic anhydride: equilibrium, kinetic and thermodynamic studies. J Radioanal Nucl Chem 315(2):195–206. https://doi.org/10.1007/s10967-017-5647-6
Qin Z, Chen Q, Lin S, Luo S, Qiu Y, Zhao L (2018) Expression and characterization of a novel cold-adapted chitosanase suitable for chitooligosaccharides controllable preparation. Food Chem 253:139–147. https://doi.org/10.1016/j.foodchem.2018.01.137
Xu J, Chen M, Zhang C, Yi Z (2013) Adsorption of uranium (VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan. J Radioanal Nucl Chem 298(2):1375–1383. https://doi.org/10.1007/s10967-013-2571-2
Elwakeel KZ, Atia AA, Guibal E (2014) Fast removal of uranium from aqueous solutions using tetraethylenepentamine modified magnetic chitosan resin. Bioresour Technol 160:107–114. https://doi.org/10.1016/j.biortech.2014.01.037
Sutirman ZA, Sanagi MM, Abd Karim KJ, Wan Ibrahim WA (2016) Preparation of methacrylamide-functionalized crosslinked chitosan by free radical polymerization for the removal of lead ions. Carbohydr Polym 151:1091–1099. https://doi.org/10.1016/j.carbpol.2016.06.076
Kyzas GZ, Siafaka PI, Lambropoulou DA, Lazaridis NK, Bikiaris DN (2014) Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for Pb(II) and Cd(II) uptake. Langmuir ACS J Surf Colloids 30(1):120–131. https://doi.org/10.1021/la402778x
Bayramoglu G, Arica MY (2016) MCM-41 silica particles grafted with polyacrylonitrile: modification into amidoxime and carboxyl groups for enhanced uranium removal from aqueous medium. Microporous Mesoporous Mater 226:117–124. https://doi.org/10.1016/j.micromeso.2015.12.040
Banerjee C, Dudwadkar N, Tripathi SC, Gandhi PM, Grover V, Kaushik CP, Tyagi AK (2014) Nano-cerium vanadate: a novel inorganic ion exchanger for removal of americium and uranium from simulated aqueous nuclear waste. J Hazard Mater 280:63–70. https://doi.org/10.1016/j.jhazmat.2014.07.026
Wu N, Li Z (2013) Synthesis and characterization of poly (HEA/MALA) hydrogel and its application in removal of heavy metal ions from water. Chem Eng J 215–216:894–902. https://doi.org/10.1016/j.cej.2012.11.084
Mittal H, Maity A, Sinha Ray S (2015) The adsorption of Pb2+ and Cu2+ onto gum ghatti-grafted poly (acrylamide-co-acrylonitrile) biodegradable hydrogel: isotherms and kinetic models. J Phys Chem B 119(5):2026–2039. https://doi.org/10.1021/jp5090857
Qiao C, Ma X, Zhang J, Yao J (2017) Molecular interactions in gelatin/chitosan composite films. Food Chem 235:45–50. https://doi.org/10.1016/j.foodchem.2017.05.045
Jridi M, Hajji S, Ayed HB, Lassoued I, Mbarek A, Kammoun M, Souissi N, Nasri M (2014) Physical, structural, antioxidant and antimicrobial properties of gelatin-chitosan composite edible films. Int J Biol Macromol 67:373–379. https://doi.org/10.1016/j.ijbiomac.2014.03.054
Hosseini M, Keshtkar AR, Moosavian MA (2016) Electrospun chitosan/baker’s yeast nanofibre adsorbent: preparation, characterization and application in heavy metal adsorption. Bull Mater Sci 39(4):1091–1100. https://doi.org/10.1007/s12034-016-1260-5
Zhao F-B, Yu Z-J, Park H-D, Liu X-Y, Song X-R, Li Z-S (2015) Polyvinylchloride ultrafiltration membranes modified with different SiO2 particles and their antifouling mechanism for oil extraction wastewater. J Environ Eng 141(8):04015009. https://doi.org/10.1061/(asce)ee.1943-7870.0000944
Yi X, He J, Guo Y, Han Z, Yang M, Jin J, Gu J, Ou M, Xu X (2018) Encapsulating Fe3O4 into calcium alginate coated chitosan hydrochloride hydrogel beads for removal of Cu (II) and U (VI) from aqueous solutions. Ecotoxicol Environ Saf 147:699–707. https://doi.org/10.1016/j.ecoenv.2017.09.036
Tonghuan L, Guojian D, Xiaojiang D, Wangsuo W, Ying Y (2012) Adsorptive features of polyacrylic acid hydrogel for UO2 2+. J Radioanal Nucl Chem 297(1):119–125. https://doi.org/10.1007/s10967-012-2316-7
Yi X, Yang M, Mo L, Xu W, Wang S, He J, Gu J, Ou M, Xu X (2018) Modification of chitosan/calcium alginate/Fe3O4 hydrogel microsphere for enhancement of Cu(II) adsorption. Environ Sci Pollut Res Int 25(4):3922–3932. https://doi.org/10.1007/s11356-017-0802-8
Şölener M, Tunali S, Özcan AS, Özcan A, Gedikbey T (2008) Adsorption characteristics of lead(II) ions onto the clay/poly(methoxyethyl)acrylamide (PMEA) composite from aqueous solutions. Desalination 223(1–3):308–322. https://doi.org/10.1016/j.desal.2007.01.221
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
He, J., Jin, J., Wang, Z. et al. Encapsulating nanosilica into polyacrylic acid and chitosan interpenetrating network hydrogel for preconcentration of uranium from aqueous solutions. J Radioanal Nucl Chem 317, 1299–1309 (2018). https://doi.org/10.1007/s10967-018-6034-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6034-7