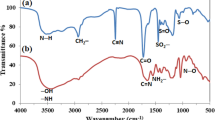
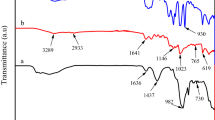
Abstract
In this study, a thermal-responsive hydrogel, sodium alginate/polyamidoxime/poly(N-isopropylacrylamide) hydrogel (SA/PAO/PNIPAm), was prepared via radical polymerization. The uranium (U) capturing properties of the hydrogel were investigated. The results reveal that SA/PAO/PNIPAm can effectively remove U(VI) from aqueous solution, and the maximal adsorption capacity was calculated to be 319.49 mg g−1 at 305.15 K and pH 5.0. Furthermore, the adsorption mechanism was revealed by X-ray photoelectron spectroscopy analysis and density functional theory simulations. In particular, high desorption efficiency was observed by changing the desorption temperature, which could reduce secondary pollutants in elution process.











Similar content being viewed by others
References
Zhang Z, Wang X, Zhou J, Zhang H, Wu F, Wu W (2022) Semi-IPN Alg/PAO microspheres for the efficient removal of U(VI) from alkaline solution by experimental and DFT study. Sep Purif Technol 296:121369
Wang X, Dai X, Shi C, Wan J, Silver MA, Zhang L, Chen L, Yi X, Chen B, Zhang D, Yang K, Diwu J, Wang J, Xu Y, Zhou R, Chai Z, Wang S (2019) A 3,2-hydroxypyridinone-based decorporation agent that removes uranium from bones in vivo. Nat Commun 10:2570
Yuan Y, Meng Q, Faheem M, Yang Y, Li Z, Wang Z, Deng D, Sun F, He H, Huang Y, Sha H, Zhu G (2019) A molecular coordination template strategy for designing selective porous aromatic framework materials for uranyl capture. ACS Cent Sci 5:1432–1439
Jin T, Luo Q, Yu H, Huang B, Liu Z, Qian Y (2023) Synergistic effects between phytic acid (PA) and urea on the extraction of uranium with porous polyvinyl alcohol (PVA) xerogel films. Sep Purif Technol 312:123367
Manos MJ, Kanatzidis MG (2012) Layered metal sulfides capture uranium from seawater. J Am Chem Soc 134:16441–16446
Liu C, Hsu P, Xie J, Zhao J, Wu T, Wang H, Liu W, Zhang J, Chu S, Cui Y (2017) A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat Energy 2:17007
Zhao G, Wen T, Yang X, Yang S, Liao J, Hu J, Shao D, Wang X (2019) Correction: Preconcentration of U(VI) ions on few-layered graphene oxide nanosheets from aqueous solutions. Dalton Trans 48:6645–6645
Bolisetty S, Mezzenga R (2016) Amyloid–carbon hybrid membranes for universal water purification. Nat Nanotechnol 11:365–371
Xiong T, Li Q, Liao J, Zhang Y, Zhu W (2022) Highly enhanced adsorption performance to uranium(VI) by facile synthesized hydroxyapatite aerogel. J Hazard Mater 423:127184
Wang Y, Li Y, Zhang Y, Zhang Z, Li Y, Li W (2021) Nanocellulose aerogel for highly efficient adsorption of uranium (VI) from aqueous solution. Carbohydr Polym 267:118233
Kuncham K, Nair S, Durani S, Bose R (2017) Efficient removal of uranium(VI) from aqueous medium using ceria nanocrystals: an adsorption behavioural study. J Radioanal Nucl Chem 313:101–112
Yan B, Ma C, Gao J, Yuan Y, Wang N (2020) An ion-crosslinked supramolecular hydrogel for ultrahigh and fast uranium recovery from seawater. Adv Mater 32:1906615
Elwakeel KZ, Atia AA (2014) Uptake of U(VI) from aqueous media by magnetic Schiff’s base chitosan composite. J Clean Prod 70:292–302
Yu S, Wei D, Shi L, Ai Y, Zhang P, Wang X (2019) Three-dimensional graphene/titanium dioxide composite for enhanced U(VI) capture: insights from batch experiments, XPS spectroscopy and DFT calculation. Environ Pollut 251:975–983
Sun Z, Chen Y, Liu Y, Na B, Meng C, Zhang S, Zou S, Liu H (2021) A proton-exchange poly(acrylic acid) supramolecular hydrogel for ultrahigh uranium adsorption. J Mater Chem A 9:21402–21409
Tan J, Xie S, Wang G, Yu CW, Zeng T, Cai P, Huang H (2020) Fabrication and optimization of the thermo-sensitive hydrogel carboxymethyl cellulose/poly(N-isopropylacrylamide-co-acrylic acid) for U(VI) removal from aqueous solution. Polymers 12:151
Ebara M, Kotsuchibashi Y, Uto K, Aoyagi T, Kim Y-J, Narain R, Idota N, Hoffman JM (2014) Smart hydrogels. Smart Biomaterials 1:9–65
Si Z, Yu P, Dong Y, Lu Y, Tan Z, Yu X, Zhao R, Yan Y (2019) Thermo-responsive molecularly imprinted hydrogels for selective adsorption and controlled release of phenol from aqueous solution. Front Chem 6:1–10
Zhu J, Luo Y, Wang J, Yu J, Liu Q, Liu J, Chen R, Liu P, Wang J (2022) Highly efficient uranium extraction by aminated lignin-based thermo-responsive hydrogels. J Mol Liq 368:120744
Yang S, Xu M, Yin J, Zhao T, Li C, Hua D (2020) Thermal-responsive ion-imprinted magnetic microspheres for selective separation and controllable release of uranium from highly saline radioactive effluents. Sep Purif Technol 246:116917
Backes S, Krause P, Tabaka W, Witt MU, von Klitzing R (2017) Combined cononsolvency and temperature effects on adsorbed PNIPAm microgels. Langmuir 33:14269–14277
Kafetzi M, Borchert KBL, Steinbach C, Schwarz D, Pispas S, Schwarz S (2021) Thermoresponsive PNIPAm-b-PAA block copolymers as “smart” adsorbents of Cu(II) for water restore treatments. Colloids Surf A Physicochem Eng Asp 614:126049
Neese F (2022) Software update: the ORCA program system—Version 5.0. Wiley Interdiscip Rev Comput Mol Sci 12:e1606
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Lu T, Chen Q (2022) Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J Comput Chem 43:539–555
Huang N, Wang J, Cheng X, Xu Y, Li W (2020) Fabrication of PNIPAm-chitosan/decatungstoeuropate/silica nanocomposite for thermo/pH dual-stimuli-responsive and luminescent drug delivery system. J Inorg Biochem 211:111216
Hu Z, Omer AM, Ouyang Xk YuD (2018) Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int J Biol Macromol 108:149–157
Wang D, Song J, Wen J, Yuan Y, Liu Z, Lin S, Wang H, Wang H, Zhao S, Zhao X, Fang M, Lei M, Li B, Wang N, Wang X, Wu H (2018) Significantly enhanced uranium extraction from seawater with mass produced fully amidoximated nanofiber adsorbent. Adv Energy Mater 8:1802607
Liu S, Zhang H, Peng D, Yuan D, Wu L, Ma J (2017) Uranium uptake with graphene oxide sponge prepared by facile EDTA-assisted hydrothermal process. Int J Energy Res 41:263–273
Wang X, Yuan L, Wang Y, Li Z, Lan J, Liu Y, Feng Y, Zhao Y, Chai Z, Shi W (2012) Mesoporous silica SBA-15 functionalized with phosphonate and amino groups for uranium uptake. Sci China Chem 55:1705–1711
Wei Y, Zhang L, Shen L, Hua D (2016) Positively charged phosphonate-functionalized mesoporous silica for efficient uranium sorption from aqueous solution. J Mol Liq 221:1231–1236
Choppin GR (2006) Actinide speciation in aquatic systems. Mar Chem 99:83–92
He N, Li H, Cheng C, Dong H, Lu X, Wen J, Wang X (2020) Enhanced marine applicability of adsorbent for uranium via synergy of hyperbranched poly(amido amine) and amidoxime groups. Chem Eng J 395:125162
Yu K, Shao P, Meng P, Chen T, Lei J, Yu X, He R, Yang F, Zhu W, Duan T (2020) Superhydrophilic and highly elastic monolithic sponge for efficient solar-driven radioactive wastewater treatment under one sun. J Hazard Mater 392:122350
Zhu W, Peng H, Luo M, Yu N, Xiong H, Wang R, Li Y (2018) Zipper-like magnetic molecularly imprinted microspheres for on/off-switchable recognition and extraction of 17β-estradiol from food samples. Food Chem 261:87–95
Zhang S, Yuan D, Zhang Q, Wang Y, Liu Y, Zhao J, Chen B (2020) Highly efficient removal of uranium from highly acidic media achieved using a phosphine oxide and amino functionalized superparamagnetic composite polymer adsorbent. J Mater Chem A 8:10925–10934
Ahmad Z, Li Y, Ali S, Yang J, Jan F, Fan Y, Gou X, Sun Q, Chen J (2022) Benignly-fabricated supramolecular poly(amidoxime)-alginate-poly(acrylic acid) beads synergistically enhance uranyl capture from seawater. Chem Eng J 441:136076
Ma C, Gao J, Wang D, Yuan Y, Wen J, Yan B, Zhao S, Zhao X, Sun Y, Wang X, Wang N (2019) Sunlight polymerization of poly(amidoxime) hydrogel membrane for enhanced uranium extraction from seawater. Adv Sci 6:1900085
Wang M, Liu H, Zeng M, Liu Y (2022) Preparation of PAMAm dendrimer modified amidoxime chelating resin and its adsorption for U(VI) in aqueous. Inorg Chem Commun 144:109909
Zhang Y, Zhang H, Liu Q, Chen R, Liu J, Yu J, Jing X, Zhang M, Wang J (2018) Polypyrrole modified Fe0-loaded graphene oxide for the enrichment of uranium(VI) from simulated seawater. Dalton Trans 47:12984–12992
Wang H, Yao H, Chen L, Yu Z, Yang L, Li C, Shi K, Li C, Ma S (2021) Highly efficient capture of uranium from seawater by layered double hydroxide composite with benzamidoxime. Sci Total Environ 759:143483
Huang G, Li W, Liu Q, Liu J, Zhang H, Li R, Li Z, Jing X, Wang J (2018) Efficient removal of uranium(VI) from simulated seawater with hyperbranched polyethylenimine (HPEI)-functionalized polyacrylonitrile fibers. New J Chem 42:168–176
Zhang H, Omer AM, Hu Z, Yang L, Ji C, Ouyang X (2019) Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu(II) adsorption. Int J Biol Macromol 135:490–500
Zhang Q, Zhao D, Feng S, Wang Y, Jin J, Alsaedi A, Hayat T, Chen C (2019) Synthesis of nanoscale zero-valent iron loaded chitosan for synergistically enhanced removal of U(VI) based on adsorption and reduction. J Colloid Interface Sci 552:735–743
Tang S, Yang J, Lin L, Peng K, Chen Y, Jin S, Yao W (2020) Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem Eng J 393:124728
Hu Z, Omer AM, Ouyang X, Yu D (2018) Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int J Biol Macromol 108:149–157
Yang X, Li J, Liu J, Tian Y, Li B, Cao K, Liu S, Hou M, Li S, Ma L (2014) Simple small molecule carbon source strategy for synthesis of functional hydrothermal carbon: preparation of highly efficient uranium selective solid phase extractant. J Mater Chem A 2:1550–1559
Acknowledgements
The authors greatly acknowledge the financial support from the National Natural Science Foundation of China (Grant Nos. 22166002, 22066004, 52162020, and 52164012), and are extremely thankful to the Fundamental Science on Radioactive Geology and Exploration Technology Laboratory (2022RGET16) for the technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known conflicts of interest that can influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, C., Meng, C., Liu, S. et al. A thermal-responsive hydrogel to capture uranium(VI) from aqueous solution: properties and mechanism. J Radioanal Nucl Chem 332, 4449–4461 (2023). https://doi.org/10.1007/s10967-023-09141-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09141-7