Abstract
222Rn is among the most important issues for radiation exposure in/near high background radiation areas such as near rare-earth-element (REE) and uranium mines in North Vietnam. Seasonal 222Rn activity concentration in spring water was determined by RAD-7, with average ranges of 1270 ± 60–66,400 ± 2630 Bq m−3, therein the highest value was a REE, and the lowest a uranium mine. The 222Rn activity concentration was higher in the dry season, which could be attributed to 222Rn leaching to spring waters from nearby mines, and lower in the rainy season due to dilution by rain water. The 222Rn annual effective doses were within permissible limits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural water, including surface water and groundwater contain a variety of beneficial elements and compounds. On the other hand, they carry significant health concerns as well, including the chances of radionuclide and heavy metal contamination. In high radioactive background areas such as those near or within rare earth element (REE) mines, uranium mines or magmatic massifs, the radionuclide activity in groundwater depends on the ability of leaching and dissolving of minerals from aquifer rocks [1, 2]. Tectonic activity, groundwater flow, weathering, natural weather and human/animal activities may lead to the release of significant amounts of radioisotopes into the surrounding environment, especially aquatic environments such as groundwater or spring water. Therefore, local residents using that water for their daily lives could be at risk as a consequence.
Radon with 3.8 days of half-life is a noble gas radionuclide, and for migration purposes it can be considered readily soluble in water [3,4,5,6]. As a gas, it has relatively high mobility, and it can move easily through fractures and migrate far from the supply [7]. Previous studies have demonstrated that among all the isotopes of the 238U decay chain, the 222Rn activity was found to be the highest in groundwater [8,9,10,11]. In that case, the principal concentration completely dissolves into groundwater without being absorbed and precipitated [10, 11]. In addition, the high radioactivity in the aquifer could have originated during movement through rocks and sediments of potentially radioactive reservoir aquifers such as phosphate, granite and black shale [12,13,14]. Groundwater or spring water can dissolve uranium and thorium minerals such as coffinite (U(SiO4)1-x(OH)4x), (UO2) and pitchblende (U3O8), which then decay to 222Rn and 220Rn. However, because the half-life of 220Rn is very short, usually 222Rn forms the main component of radioactive gas in water or groundwater [15]. Therefore, the radon contamination potential in spring water as well as underground water sources at or close to the high natural radioactive background areas are areas of research interest. It should be mentioned that gross alpha and beta measurements in drinking water are a screening tool in still in favor with many authorities, however such measurement have some limitations, so radionuclide specific measurements can be preferred [16].
222Rn can cause undesirable effects on public health directly upon drinking of radon-rich water for a long time or indirectly by increasing the indoor radon concentration. The decay products of radon such as 210Pb and 210Po if inhaled can cause lung cancer and tissue damage [17, 18], being the reason for thousands of cancer deaths each year [19]. Similarly ingesting radon rich water leads to the increase of intestinal cancers [20].
The WHO guidelines of 222Rn concentration in drinking water recommend less than 100 Bq L−1 as acceptable [20]. Similarly, European Union recommendation is that concentrations below 100 Bq L−1 require no remedial action, while above 1000 Bq L−1 action is radiologically warranted. Recent efforts to detect and evaluate the effects of consuming 222Rn containing water in different aquatic environments helped in implementing strategies decreasing their impact on public health [19, 21].
There are multiple REEs and uranium mineral concentrations located in the North–Viet Nam region [22]. Research studies pertaining to the radioactivity in various environmental media were carried out in and surrounding those areas, however there is only limited information on radon in the local water resources available [23,24,25,26,27,28,29].
In high mountainous areas, the source of drinking water is mainly rain, well and spring water (the majority is spring water). Spring water in these areas has been identified as close to or flowing past REEs and Uranium mines, which increases the possibility of it harboring high radioactivity concentrations of 222Rn and its parents, 226Ra and 238U. [30]. Therefore, local people may be exposed to high levels of internal radiation when they use that water source for living and drinking. Determining the concentration and radiological hazard assessment for the consumption of 222Rn containing spring water in the high natural radioactive background level areas is an important part of assuring public health in the area. The results will provide a database for radioactive dose assessment and will aid in assessing the impact of mining activities due to the release of 222Rn radionuclides into the surrounding environment. In addition, this present study also presents the seasonal variation of 222Rn activity and emphasizes the influence of climatic factors on the distribution and content of 222Rn in spring water sources in North Vietnam.
Material and method
Study area
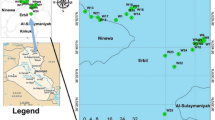
North Vietnam was affected by many tectonic and magmatic events that were sources of ore and natural radionuclide deposits (rare earth elements (REEs) and uranium mines) [31,32,33,34,35]. The REEs mines in North–Vietnam [36] include the Muong Hum—Lao Cai, Nam Xe—Lai Chau, Dong Pao—Lai Chau, and Yen Phu—Yen Bai REE mines, while uranium mines include Ta Xua—Son La, Dong Cuu—Phu Tho and Binh Duong—Cao Bang as presented in (Fig. 1).
Study areas (modified from Duong et al. [36])
The Nam Xe—Lai Chau mine is the largest with about 7.7 million tons of REEs reserve in Vietnam [37]. The U, Th potential of this area is also relatively large, with a reserve of about 76,000 tons of U3O8, 59,000 tons of ThO2 in the north of the mine [36]. The second largest REE mine is Dong Pao- Lai Chau mine with a reserve of 3.7 million tons, where the U and Th concentrations in the ore were reported to be 0.3 and 0.01%, respectively [24]; the Muong Hum—Lao Cai mine has a reserve of about 0.4 million ton of REE, the U3O8 and ThO2 concentrations were recorded as 0.17—0.15% and 0.08—0.03%; the Yen Phu—Yen Bai mine has a REE reserve about 4000 tons, with ThO2 and U3O8 concentrations about 0.001–0.383 and 0.001–0.04%, respectively [1, 38]. The Ta Xua—Son La, Dong Cuu—Phu Tho and Binh Duong—Cao Bang uranium mines have concentrations of U3O8 approximately 0.05; 0.05–0.06 and 0.05–0.06%, respectively.
Nam Xe and Dong Pao from Lai Chau are over the Fa Si Pan Granite formation [39], and while Muong Hum from Lao Cai is over a Paleozolic magmatic rock formation, that is partially covered by thin bedded limestone, clayey limestone, clay shale and calcareous sandstone [40]. Yen Phu from Yen Bai lies over a Quaternary sedimentary rock formation, however due to the vicinity of the Red River fault the aquifer includes areas influenced by magmatic activity [39]. Ta Xua from Son La is located over Permian–Triassic sedimentary rocks [39]. Dong Cuu from Phu Tho lies over the Thach Khoan Formation mainly of mica quartz schist, mica schist, staurolite-bearing quartz, disten, sillimanite, and garnet [41]. Finally, Binh Duong from Cao Bang lies over mixed Triassic sedimentary rocks [42].
Method
Monitoring the variation in 222Rn activity in spring water was conducted for both dry and rainy seasons. North Vietnam is characterized by a tropical monsoon climate with high temperature and rainfall in summer (wet or rainy season) and low temperature and rainfall in winter (dry season). The rainy season runs from May to September or October and the dry season runs from October through May. Water samples were evaluated for radon activity at August for the rainy season and in December for the dry season. A total of 126 samples were collected from spring streams flowing from the alpine terrain at seven locations. At each location, the sampling was carried out for 3 consecutive days. Each day the samples were taken at 3 different times including the morning, noon, and evening. We have selected typical sampling sites at locations used by local residents, nearby uranium and rare earth element mining activities. The 222Rn activity concentration was directly measured using a RAD 7 m manufactured by Durridge Company Inc (USA) combined with the RAD H2O kit, with a measuring range from 0.1 to 200,000 pCi L−1 and accuracy of 0.1 pCi L−1 (3.7–740 Bq L−1 with an accuracy of 3.7 mBq L−1), using a continuous loop aeration system, a technique commonly used in similar studies on drinking water [43], ground water and spring water [44].
To get an accurate performance, the experimental procedure was conducted according to the manufacturer’s instructions [45], using the 250 mL and 40 mL plastic vials from the RAD H2O kit depending on activity concentration. The detection limit using the 250 mL vial was 0.037 Bq L−1 (37 Bq m−3) using a 300 min count, while for the 40 mL vial it was 0.259 Bq L−1 (259 Bq m−3) using a 300 min count.
Annual effective dose (AED)
The AED to humans contributed by 222Rn from water consumption is determined by the formula below.
The AED from ingestion was determined by formula (1).
where
-
CRn is the 222Rn activity average in water (Bq L−1).
-
Df is the 222Rn dose conversion factor (mSv Bq−1), the values for adult (> 17 years), child (> 7 to 12 years) and infant (1–2 years) are 3.5 × 10−6, 5.9 × 10−6, and 23 × 10−6 mSv Bq−1 respectively [46].
-
Ai is the annual water consumption (according WHO (2008) the annual water consumption for adults, children and infants is 720, 330 and 230 L, respectively [20]).
The AED from inhalation was determined by formula (2):
where
-
CRn is the average 222Rn activity in water sample (Bq L−1),
-
R is the ratio between 222Rn in air and water (10–4),
-
F is the equilibrium coefficient between 222Rn and its progenies (0.4),
-
Df is the 222Rn dose conversion factor equal to 9 × 10–6 mSv Bq−1 h−1 m.3 [46]
-
T: the average indoor time of each individual (7000 h/year) [46, 47].
Formula 2, while commonly used for evaluating the effects of radon in water for the increase of inhalation dose is, in the current study, an overestimation of the actual risk. The exposure scenario in the formula is that water is piped in the house, where the degassing radon increases indoor radon concentration, leading to the increase of lung cancer risk, assuming people spend approximately 80% of their time indoors. Thus it is only applicable when water is piped inside, if water is collected at the spring, then part of the radon never enters the house, however it could provide a conservative upper estimate.
Result and discussion
222 Rn activity concentration
The 222Rn activity concentrations in spring water at different areas are presented by season in Table 1 and Fig. 2. The 222Rn activity concentration in the dry season ranged from 1640 ± 80−3 to 89,900 ± 3550 m−3. The values recorded during the rainy season were significantly lower than in the dry season, where the 222Rn activity concentration in spring water was 900 ± 40–42,900 ± 1700 m−3. These values are lower than the recommended international upper limit of 100 Bq L−1 (100,000 Bq m−3) [20], so they can be considered safe to drink in regards to radon activity concentration, the influence of the mines, if any, is limited. The 222Rn activity ratio between dry and rainy seasons was calculated to range from 1.5 to 2.7 with an average value of 1.9. This may be useful information for interpreting and comparing literature data in the area. There were significant differences in the yearly average of 222Rn activity concentration in spring water in the different regions as well. The 222Rn activity concentration ranged from 1270 ± 60 m−3 (Binh Duong—Cao Bang) located over sedimentary rocks to 66,400 ± 2630 m−3 (Muong Hum-Lao Cai) located over a Paleozolic magmatic rock formation, partially covered by limestone, clay and other sediments. The highest value in the Muong Hum-Lao Cai mine area could be related to the spring activity in the sampling site, where spring water passed through the Muong Hum-Lao Cai REE mine.
The 222Rn activity concentration difference in spring water at different areas can be due to multiple causes, such as differences in aquifer geology [48, 49], regional tectonic and magmatic activities [35, 50,51,52] or physicochemical properties of water source [46, 53]. Also the nature of the mines in the area might be a factor, REE mines tend to be surface mines, more easily influenced by erosion and weathering than the uranium mines, which tend to be deep mines. Previous studies suggested that the 222Rn activity concentration in the water source was controlled mainly by the concentration of its parent isotope 226Ra [9, 10, 19, 54, 55]. The 226Ra spatial distribution was an important factor [56]. However previous studies with sampling points located in the study area found no statistically significant differences between 226Ra activity concentration in well water and soil samples [25, 26]. On the other hand, the 222Rn concentration in the aquifers or surface water was reported to depend on mainly the leaching and dissolution from the host formation in multiple sources [18, 44, 57]. The presence of 222Rn may be related to physicochemical properties of water source, or tectonic activity. This research gap needs to be further studied to accurately determine the origin and influencing factors on the distribution of 222Rn activity concentration in spring water in the studied areas.
In all of the study areas, the average 222Rn concentration in water was lower than the EU reference value of maximum 100 Bq L−1 (100,000 Bq m−3) [58], and can be considered safe to drink in this regard. Previous research by Le Khanh (2015) showed that the 222Rn concentration in the air was determined to be significantly higher than the world average at Nam Xe—Lai Chau mine (reaching 300 Bq L−1 or 300,000 Bq m−3) [23]. The 222Rn concentration determined in the air was directly influenced by mining activities leading to the dispersion of radon into the surrounding environment [23]. Since the ratio of 222Rn in air and in water used for calculating the effects of radon in water is 10–4 according to Duggal et al. and UNSCEAR [43, 46], our results indicated that there is a limited influence of mining activities on the distribution and dispersion of 222Rn through spring water in study area opposed to the significant influence it had at Nam Xe [23].
Seasonal variation of radon concentration in spring water
The variation of 222Rn concentration in groundwater and surface water over time has been of interest to scientists for a long time [9, 54, 59]. The climate was identified as one of the most important factors affecting the 222Rn concentration distribution in groundwater and surface water [46, 53]. Lower air temperature could significantly reduce water temperatures and increase the solubility of radon [60,61,62]. The solubility of a gas depends on temperature and could be expressed as Cg related to the pressure of the undissolved gas above the solution through the k proportionality constant, which relation could be distorted if a chemical reaction takes place [62]. The radon solubility in water decreases and it escapes rapidly at higher temperatures [63]. In addition, there was an inverse correlation observed between 222Rn content in water source and precipitation (rainfall) [64, 65], likely caused by dilution due to heavy rainfall. The 222Rn activity concentration in water was reported to increase in winter (dry season) and decrease in summer (rainy season) [9, 54, 59].
Seasonal 222Rn activity concentration monitoring in this study showed that activity concentrations in spring water were comparatively high in the dry season and low in the rainy season. The climate in the North of Vietnam is characterized by a tropical monsoon climate with high temperature and rainfall in summer (rainy season) and low temperature and rainfall in winter (dry season). As it can be seen on Fig. 3, there was strong positive relationship between the 222Rn concentration in spring water in the dry and rainy seasons (with a coefficient of determination R2 = 0.99 and a Pearson correlation coefficient of R = 0.99, the data passes the Kolmogorov–Smirnov test of normality, and the strong correlation persist, with an R = 0.86 or greater, if we remove any or all of the high concentration values, the good correlation is not falsely caused by outliers, however it must be admitted that the dataset is limited to seven locations, with each dot representing the average value of 3 measurements per day on three consecutive days). This could potentially make it easier to interpret and compare literature data, and plan monitoring campaigns.
The trend of seasonal 222Rn content variation in spring water in this study seems to be consistent with the results of previous studies [9, 54, 59]. Changes in aquifers can lead to disruption of recharge sources, which might cause corresponding changes in 222Rn activity concentration fluctuations. Geological structures play an important role in radon transport [18]. Tectonic activities can lead to changes in aquifer properties such as porosity and permeability. These properties are among the factors influencing 222Rn migration and dispersal capacity and the recharge rate of water sources [66,67,68]. Therefore, the application of 222Rn activity concentration monitoring could become a tool for not only radiation hazard studies, but can also be incorporated to analyses and prediction of the recharge rate of water sources, monitoring climate change and geological hazards, in order to solve the environmental issues present in these study areas.
Assessment of the AED
The AED for 222Rn in this study was determined by Formulas 1 and 2 and presented in Table 2 and Table 3.
People who consume water sources containing 222Rn will get internal exposure, which can be estimated by the annual effective dose. Overall, the average AED for adults, children and infants can be considered acceptable, with values ranging from 3.2 to 167.5 and 37.9 µSv y−1 on average for adults; from 2.5 to 129.4 and 29.3 µSv y−1 on average for children and from 6.7 to 351.4 and 79.6 µSv y−1 on average for infants. Despite consuming less water, infants were found to have significantly higher AED than adults, due to the higher dose conversion coefficient. This difference was attributed to a more vigorous metabolism and lower organ mass compared with adults [43, 46]. This value could be an overestimation, since infants generally should not receive untreated water, and boiling the water would reduce the radon concentration in water.
Table 3 shows that the expected excess AED from inhaling 222Rn released from spring water is similar to that of ingestion for adults, however based on the actual conditions in the area, this is an overestimation, actual doses are expected to be less if water is not piped into houses. The arithmetical mean over the whole region is 37.8 µSv y−1. The maximum 167.3 µSv y−1 was calculated for the Muong Hum-Lao Cai mine area.
Considering both pathways for radon exposure from spring water the natural sources and the mining activity combined results in radon activity concentrations in spring water that are considered acceptable by international guidelines.
Conclusions
The 222Rn activity concentration in spring water samples close to seven REEs and uranium mines were investigated in North Vietnam. Based on given results, some conclusions were drawn as follows:
The various 222Rn activity concentration levels dissolved in spring water sources were lower than the WHO and EU reference levels for drinking water. The yearly average 222Rn activity concentration was recorded ranging from 1270 ± 60 Bq m−3 (Binh Duong—Cao Bang) to 66,400 ± 2630 Bq m−3 (Muong Hum—Lao Cai) with an average value of 15,030 ± 620 Bq m−3. Spring water in the vicinity of two rare earth element mines and one uranium mine had radon levels much higher than typical for surface water, however it did not reach 100 Bq L−1 (100,000 Bq m−3), so the risk is considered acceptable by most international recommendations, the spring waters are safe to drink in this regard.
The seasonal 222Rn content variation in the spring water sources showed to be higher in the dry season (winter), approximately double, and lower in the rainy season (summer) with the average activity concentration values of 20,400 ± 800 Bq m−3 and 9700 ± 400 Bq m−3, respectively. The good correlation might have implications for interpreting literature data and planning monitoring activities, however caution is advised against interpolating based on a limited dataset. The physicochemical factors affecting the 222Rn activity concentrations and distribution in the studied spring water sources need to be further studied in detail.
The AED for local residents who consume the spring water resources is within the acceptable range, with mean values determined to be 37.9 µSv y−1 for adults, 29.3 µSv y−1 for children and 79.6 µSv y−1 for infants, respectively. The dose for infants is probably overestimated, due to boiling water reducing the actual radon concentration in the water intended for consumption. The AED increase from inhaling excess radon degassing from water is probably less than 37.8 µSv y−1 on average over the whole region, also within the acceptable range.
The concentration of other radionuclides, such as 238U, 226Ra, 228Ra, 210Pb and 210Po should also be checked in water resources in the area to assure radiological safety. Based on our results, the effect of mining on the concentration 222Rn in spring water in the area and the consequent probable health risk are relatively low, despite previous reports on mining significantly impacting 222Rn activity concentrations in air.
Change history
21 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10967-023-08995-1
References
Nguyen TD, Duong VH, Duong DT, Phan TT, Le Xuan H (2021) Natural radionuclides and assessment of radiological hazards in Muong Hum, Lao Cai, Vietnam. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128671
Dung NV, Anh VTL (2021) Natural radioactivity and environmental impact assessment at Dong Pao rare earth mine, Lai Chau, Vietnam. Springer, Berlin, Heidelberg
Ramola RC, Choubey VM, Negi MS, Prasad Y, Prasad G (2008) Radon occurrence in soil–gas and groundwater around an active landslide. Radiat Meas. https://doi.org/10.1016/j.radmeas.2007.05.054
Kumar A, Kaur M, Sharma S, Mehra R (2016) A study of radon concentration in drinking water samples of Amritsar city of Punjab (India). Radiat Prot Environ. https://doi.org/10.4103/0972-0464.185155
Inácio M, Soares S, Almeida P (2017) Radon concentration assessment in water sources of public drinking of Covilhã’s county. J. Radiat. Res. Appl. Sci, Portugal. https://doi.org/10.1016/j.jrras.2017.02.002
Keramati H, Ghorbani R, Fakhri Y, Khaneghah AM, Conti GO, Ferrante M, Ghaderpoori M, Taghavi M, Baninameh Z, Bay A, Golaki M, Moradi B (2018) Radon 222 in drinking water resources of Iran: a systematic review, meta-analysis and probabilistic risk assessment (Monte Carlo simulation). Food Chem Toxicol. https://doi.org/10.1016/j.fct.2018.03.042
Lindsey BD, Ator SW (1996) Radon in ground water of the lower susquehanna and potomac river basins. US Department of the Interior, US Geological Survey
Hess CT, Michel J, Horton TR, Prichard HM, Coniglio WA (1985) The occurrence of radioactivity in public water supplies in the United States. Health Phys. https://doi.org/10.1097/00004032-198505000-00002
Loomis DP, Watson JE, Crawford-Brown DJ (1988) Predicting the occurrence of radon-222 in groundwater supplies. Environ Geochem Health. https://doi.org/10.1007/BF01758591
Baskaran M (2016) Radon in groundwater system. Radon: a tracer for geological geophysical and geochemical studies. Springer, Berlin, Heidelberg, pp 167–188
IAEA (2014) The environmental behaviour of radium: revised edition http://www-pubiaeaorg/MTCD/Publications/PDF/trs476_webpdf
Shin W, Oh J, Choung S, Cho BW, Lee KS, Yun U, Kim HK (2016) Distribution and potential health risk of groundwater uranium in Korea. Chemosphere. https://doi.org/10.1016/j.chemosphere.2016.08.021
Gascoyne M (1992) Geochemistry of the actinides and their daughters. In Uranium-series disequilibrium: applications to earth, marine, and environmental sciences sciences 2 ed
Ayotte JD, Flanagan SM, Morrow WS (2007) Occurrence of uranium and 222radon in glacial and bedrock aquifers in the Northern United States. US Department of the Interior, US Geological Survey, p 84
Jalili MA, Behtash A, Rezaei-Ochbelagh D (2012) Radon concentration in hot springs of the touristic city of Sarein and methods to reduce radon in water. Radiat Phys Chem. https://doi.org/10.1016/j.radphyschem.2012.03.015
Eaton A, Shannon R (2015) Radionuclide rule compliance: utility guidance on analytical methods, American water works association https://www.awwa.org/Portals/0/AWWA/ETS/Resources/Technical%20Reports/RadionuclideAnalyticalMethodsGuide.pdf?ver=2021-05-21-121356-470
USEPA (1999) National primary drinking water regulations, Radon-222 Federal Register US Environmental Protection Agency November 2, 1999, 64(211)
Sukanya S, Noble J, Joseph S (2021) Factors controlling the distribution of radon (222Rn) in groundwater of a tropical mountainous river basin in southwest India. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128096
Telahigue F, Agoubi B, Souid F, Kharroubi A (2018) Groundwater chemistry and radon-222 distribution in Jerba Island, Tunisia. J Environ Radioact 182:74–84. https://doi.org/10.1016/j.jenvrad.2017.11.025
WHO, 2008 Guidelines for drinking-water quality, incorporating the first and second addenda (3rd ed), vol 1 Recommendations, World Health Organization, Geneva, Switzerland
Qadir RW, Asaad N, Qadir KW, Ahmad ST, Abdullah HY (2021) Relationship between radon concentration and physicochemical parameters in groundwater of Erbil city Iraq. J Radiat Res Appl Sci. https://doi.org/10.1080/16878507.2020.1856588
Tu CM, Huan TD, Hoa NV, Ton ND, Long ND, Phong DH (2013) Characteristics of rare earth ore mineralization in South Nam Xe Area. Lai Chau Province J Geol A 335:67–76
Le KP, Bui DD, Nguyen DC, Kovács T, Nguyen VN, Duong VH, Vu TML (2015) Estimation of effective dose rates caused by radon and thoron for inhabitants living in rare earth field in northwestern Vietnam (Lai Chau province). J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-014-3881-8
Chau ND, Jadwiga P, Adam P, Van Hao D, Le Khanh Phon JP (2017) General characteristics of rare earth and radioactive elements in Dong Pao deposit, Lai Chau, Vietnam. Vietnam J Earth Sci 39(1):14–26
Duong VH, Nguyen TD, Hegedus M, Kocsis E, Kovacs T (2021) Study of well waters from high-level natural radiation areas in Northern Vietnam Int. J Environ Res Public Health. https://doi.org/10.3390/ijerph18020469
Duong VH, Nguyen TD, Kocsis E, Csordas A, Hegedus M, Kovacs T (2021) Transfer of radionuclides from soil to Acacia auriculiformis trees in high radioactive background areas in North Vietnam. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2021.106530
Phan QV, Dao TT, Nguyen P, Trinh DH, Heinig T (2019) An assessment of natural radioactivity in the Namxe rare earth deposit, Laichau Province Vietnam. Minerals 9(10):602. https://doi.org/10.3390/min9100602
Nguyen DC, Nowak J (2021) Natural radioactivity in thermal waters: a case study from Poland. Energies. https://doi.org/10.3390/en14030541
Nguyen DC, Le Khanh P, Jodłowski P, Pieczonka J, Piestrzyński A, Duong VH, Nowak J (2016) Natural radioactivity at the sin quyen iron-oxide-copper-gold deposit in North Vietnam. Acta Geophys. https://doi.org/10.1515/acgeo-2016-0103
Van Duong H, Nguyen CD, Nowak J, Kovacs T, Hoang QA (2019) Uranium and radium isotopes in some selected thermal, surface and bottled waters in Vietnam. J Radioanal Nucl Chem 319:1345–1349
Dung PT, Anh TT, Hung TQ, Hoa TT, Shelepaev RA, Hoang N, Cong TQ (2021) Petrographic and geochemical characteristics of the Nui Chua pegmatoid mafic-ultramafic series, Northern Vietnam: significance in petrogenesis and Fe-Ti-V metallogenesis. Sci Earth 43(1):81–95
Hoang N, Huong TT, Bac DT, Van Vu N, Thu NT, Thang CS, Dang PT (2016) Magma source feature and eruption age of volcanic rocks in the Tram Tau district, Tu Le Basin. Vietnam J Earth Sci 38(3):242–255. https://doi.org/10.15625/0866-7187/38/3/8710
Dung PT, Hoa TT, Anh TT, Hieu PT (2019) Formation pressure-temperature (PT) of Ye Yen Sun granite. Sci Earth 41(2):173–181. https://doi.org/10.15625/0866-7187/41/2/13735
Trinh DH, Luu Cong T, Nguyen Tuan A, Tran Viet A, Phan Hoang G, Takahashi S (2021) Paleogene granite magmatism in the north of the Truong Son belt and implication for crustal evolution. Vietnam J Earth Sci. https://doi.org/10.15625/2615-9783/16444
Findlay RH (2018) Geometry, kinematics and regional significance of faulting and related lamprophyric intrusion in the mineralised zone at the Pu Sam Cap complex Northwest Vietnam. Sci Earth 40(4):320–340
Yolanda FS (2013) The mineral industry of Vietnam US geological survey minerals yearbook 2011. http://mineralsusgsgov/minerals/pubs/country/2011/myb3-2011-vnpdf Accessed 02 Aug 2014
Moody MD (2013) Mother lode: The untapped rare earth mineral resources of Vietnam. NAVAL WAR COLLEGE NEWPORT RI JOINT MILITARY OPERATIONS DEPT
Luong Quang Khang (2012) Characteristics of mineralization of the Yen Phu rare earth deposit, Yen Bai Province
Roger F, Maluski H, Lepvrirer C, Van TV, Paquette J-L (2012) LA-ICPMS zircons U/Pb dating of Permo-Triassic and Certaceous magmatisms in Northern Vietnem—Geodynamical implications. J Asian Earth Sci 48:72–82. https://doi.org/10.1016/j.jseaes.2011.12.012
Dung NV, Thuan DD, Nhan DD, Carvalho FP, Thang DV, Quang NH (2022) Radiation exposure in a region with natural high background radiation originated from rare earth element deposits at Bat Xat district. Vietnam Radiat Environ Biophys 61:309–324. https://doi.org/10.1007/s00411-022-00971-9
Bac BH, Dung NT, Khang LQ, Hung KT, Lam NV, An DM, Son PV, Anh TTV, Chuong DV, Tinh BT (2018) Distribution and characteristics of nanotubular Halloysites in the Thach Khoan Area, Phu Tho Vietnam. Minerals 8(7):290. https://doi.org/10.3390/min8070290
Wysocka A, Pha PD, Durska E, Czarniecka U, Thang DV, Filipek A, Cuong NQ, Tuan DM, Huyen NX, Tha HV (2018) New data on the continental deposits from the Cao Bang Basin (Cao Bang-Tien Yen Fault Zone, NE Vietnam)—biostratigraphy, provenance and facies pattern. Acta Geol Pol 68(4):689–709. https://doi.org/10.1515/agp-2018-0037
Duggal V, Sharma S, Mehra R (2020) Risk assessment of radon in drinking water in Khetri Copper Belt of Rajasthan India. Chemosphere 239:124782. https://doi.org/10.1016/j.chemosphere.2019.124782
Moreno V, Bach J, Zarroca M, Font L, Roqué C, Linares R (2018) Characterization of radon levels in soil and groundwater in the North Maladeta Fault area (Central Pyrenees) and their effects on indoor radon concentration in a thermal spa. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2018.03.001
RAD H2O User Manual, 2018 Radon in water accessory for the RAD7 Durridge radon capture & analytics
United Nations Scientific Committee on the Effects of Atomic Radiation (2000) Sources and effects of ionizing radiation, ANNEX B, Exposures from natural radiation sources UNSCEAR 2000 REPORT, New York, 1, 97-99
ICRP, 1993 International Commission on Radiological Protection Protection against radon-222 at home and at work ICRP publication 65 Ann ICRP 23 (2), 1–38, 1993
Gunderson LCS (1988) Correlation between geology, radon in soil gas, and indoor radon in the reading prong. Geol Causes Nat Radionucl Anom. https://doi.org/10.1007/BF02627832
Appleton JD, Miles JCH, (2005) Radon in wales In: Basset, MG, Deisler, VK, Nichol, D (Eds), Urban Geology of Wales 2, (vol 24) National Museum of Wales Geological, Cardiff, pp 117–130
Xuan PT, Van Pho N, Van Chinh V, Dang PT, Lien NT, Tra DT, Nga HT, Van Quynh B, Van Luan N, Qua NX (2017) Study on active tectonic faults using soil radon gas method in Viet Nam. Sci Earth 39(1):27–46
Trinh PT, Liem NV, Huong NV, Vinh HQ, Thom BV, Thao BT, Tan MT, Hoang Ng (2012) Late quaternary tectonics and seismotectonics along the Red River fault zone North Vietnam. Earth Sci Rev. https://doi.org/10.1016/j.earscirev.2012.06.008
Trinh PT, Vinh HQ, Huong NV, Liem NV (2013) Active fault segmentation and seismic hazard in Hoa-Binh reservoir Vietnam. Centr Eur J Geosci 5:223–235. https://doi.org/10.2478/s13533-012-0128-5
Akawwi E (2014) Radon-222 concentration in the groundwater along eastern Jordan rift. J Appl Sci. https://doi.org/10.3923/jas.2014.309.316
Seminsky KZ, Seminsky АK (2019) Radon concentration in groundwater sources of the Baikal region East Siberia Russia. Appl Geochem 111:104446. https://doi.org/10.1016/j.apgeochem.2019.104446
Deveaud S, Millot R, Villaros A (2015) The genesis of LCT-type granitic pegmatites, as illustrated by lithium isotopes in micas. Chem Geol 411:97–111. https://doi.org/10.1016/j.chemgeo.2008.10.022
Przylibski TA (2000) Estimating the radon emanation coefficient from crystalline rocks into groundwater. Appl Radiat Isot 53(3):473–479. https://doi.org/10.1016/S0969-8043(99)00145-1
Przylibski TA (2011) Shallow circulation groundwater–the main type of water containing hazardous radon concentration. Nat Hazards Earth Syst Sci. https://doi.org/10.5194/nhess-11-1695-2011
Council Directive 2013/51/Euratom of 22 October 2013 Laying Down Requirements for the Protection of the Health of the General Public with Regard to Radioactive Substances in Water Intended for Human Consumption Official Journal of the European Union
Rao NS, Sengupta D (2010) Seasonal levels of radon and thoron in the dwellings along southern coastal Orissa Eastern India. Appl Radiat Isot 68(1):28–32. https://doi.org/10.1016/j.apradiso.2009.09.026
Schubert M, Paschke A, Lieberman E, Burnett WC (2012) Air–water partitioning of 222Rn and its dependence on water temperature and salinity. Environ Sci Technol. https://doi.org/10.1021/es204680n
Erdogan M, Eren N, Demirel S, Zedef V (2013) Determination of radon concentration levels in well water in Konya Turkey. Radiat Protect Dosim 156(4):489–494. https://doi.org/10.1093/rpd/nct099
Clever HL (1985) Krypton xenon radon gas solubilities. Solubility data series. Pergamon Press, Oxford
Wilhelm E, Battino R, Wilcock RJ (1977) Low-pressure solubility of gases in liquid water. Chem Rev. https://doi.org/10.1021/cr60306a003
Andrews JN, Wood DF (1972) Mechanism of radon release in rock matrices and entry into groundwaters Bath Univ of Tech, Eng
Fukui M, Katsurayama K (1983) The 222Rn content of groundwater in strata of the Plio-Pleistocene Osaka group in the Sennan area Japan. J Hydrol. https://doi.org/10.1016/0022-1694(83)90022-7
Al-Bataina BA, Al-Taj MM, Atallah MY (2005) Relation between radon concentrations and morphotectonics of the Dead Sea transform in Wadi Araba Jordan. Radiat Meas 40(2–6):539–543. https://doi.org/10.1016/j.radmeas.2005.06.023
Shi X, Hoftiezer DJ, Duell EJ, Onega TL (2006) Spatial association between residential radon concentration and bedrock types in New Hampshire. Environ Geol. https://doi.org/10.1007/s00254-006-0304-3
Li C, Su H, Zhang H, Zhou H (2016) Correlation between the spatial distribution of radon anomalies and fault activity in the northern margin of West Qinling Fault Zone, Central China. J Radioanal Nucl Chem 308:679–686. https://doi.org/10.1007/s10967-015-4504-8
Funding
Open access funding provided by University of Pannonia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duong, VH., Vu, HD., Nguyen, D.T. et al. Seasonal 222Rn activity in spring water close to rare earth element and uranium mines in North Vietnam. J Radioanal Nucl Chem 333, 2537–2545 (2024). https://doi.org/10.1007/s10967-023-08872-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08872-x