Abstract
Promising green leaching technique was used by Humic acid (HA) for removing uranium from Abu Zeneima spent residue for environmental safety and cost-effective leaching. The studied residue is outlet from vat leaching process using sulfuric acid leaching of carbonaceous shale ore material with initial uranium assays 185 ppm, which representing a hazardous waste. The overall leaching efficiency assaying 93% of uranium using humic acid leaching at curing temperature 70 °C, 13% HA with S/L ratio of 1/1.5 for 15 day. Kinetic study of leaching process proved diffusion controlling mechanism with activated energy 10.297 kJ/mol. Finally; 98% of uranium was extracted using Amberlite IRA- 400 resin with purity of 97.3%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uranium processing is associated with a wide range of potential adverse human health risks. The side effect of uranium residue in mining sites and waste treatment operations is the radon decay products which represent the greatest radiation-related health risk, Radon’s alpha-emitting radioactive decay products is strongly and causally linked to lung and bones cancer for humans. The radionuclides is a greatest health-related concern in uranium processing are those present in the (238U), (235U), and (232Th) decay series, numerous factors are effect on release radiation including the type of ore deposit, uranium grade, mineralogy of deposit, variation in process, reagents used for the chemical dissolution of uranium-bearing mineral species, solid–liquid separation method, purification method, precipitation and waste accumulation [1]. In this context, it can be stated that, the radioactive wastes were not only accumulate as a result of uranium mining, but also during its processing resulted in release of radioactivity into the environment and soils [2, 3]
Solvent extraction is one of standard process that currently used for the removing of metal ions with a high selectivity [4,5,6,7], this technique was especially used for the high metal concentration as the same as precipitation technique. The most efficient function groups in the solvent extraction are that based on phosphorus moieties as alkyl phosphates [5, 8,9,10,11,12], and phosphoric acid derivatives [13]. A wide variety of functional groups characterized by a high affinity but also selectivity toward uranium even in low concentration from high saline solution. Among of these groups; amidoxime groups on synthetic and biopolymer composites [14,15,16,17], metal organic framework [18], sulfonic functionalized materials [16, 19,20,21], quaternary ammonium groups based resin [22,23,24,25,26], and iminodiacetic-bearing groups [27]. A great attention in the last decades has been focused on the modification and developments of highly adsorptive uranium composites for enhancing the uranium recovery from industrial effluents, mining solutions and sea water [28,29,30,31,32,33], as well as the beneficial effects of bifunctional composites for uranium adsorption [34,35,36,37,38,39,40,41].
Generally, uranium production was achieved via either conventional and\or non-conventional leaching techniques using mineral acids and/or alkaline reagents processes for the former, and organic acids such as oxalic, citric, fulvic or humic acids for the latter [42, 43]. Humic acids (HA) are considered as a high-molecular-weight organic substance. It is soluble in alkaline media, but insoluble in acidic media [44,45,46]. It is worthy to mention herein that, Humic acids have an acidic character. The carboxyl groups are strongly acidic with dissociation constant in the range of 102–105, while OH groups have a dissociation constant in the range of 109–1011 [47]. HA have an affinity to make complexation with heavy and rare metals, as well as actinides. This complexation is an important factor that influences the precipitation or migration behavior of high valent metal ions besides adsorption and oxidation–reduction process[48, 49]. The complex equilibrium between uranium and Humic substances was achieved at approximately 72 h with coordination number varied from 1:1 to1:2 U (VI): HA as pH increased from 3.0 to 6.0. The stability constant of complexes decreased with increasing temperature, but also increased with increasing pH value [50]. Also, De Melo [51], and Chinese [3] are reported that a large number of possible reactions and interactions of uranium with HA which depends on the pH and cation concentration of the leach liquors, the functional group and the degree of saturation of the potential sorption sites.
On the other hand, HA can effectively interact with pollutants, through sorption or covalent bond formation and thus affect their mobility and transformation in soil. HA can reversibly bind cations and non-ionic compounds, including organic pollutants, by means of unspecific interaction processes, such as electrostatic interactions, hydrogen bonds and dipole interactions [52, 53].
In Egypt, several previous studies were focused upon the recovery of uranium (U) and the associated valuable elements e.g., Cu, Zn, Ni, REE, etc. from Abu Zeinema uraniferous ore material of west central Sinai area, using different leaching methods [54,55,56,57,58,59,60]. Also, from the environmental point of view, the spent residue produced from Abu Zeinema pilot plant have received special interest to be managed and disposed of safely.
Accordingly, the present work was directed to study the recovery of U from Abu Zienema pilot plant hazardous spent residue using HA as a green lixiviate due to environmental concerns. The working sample was collected from the solid spent residue pile produced after sulfuric acid leaching process. It was then specified and processed to study kinetics of leaching process as well as the extraction of U content.
Experimental
Materials and chemical reagents
The working sample of the present study is a residue stored after acidic treatment (i.e., 5% H2SO4) of carbonaceous shale ore material in Abu Zienema pilot plant. It is noteworthy that the sample with particle size about (− 100) mesh was firstly dried at room temperature. Humic acid was purchased from Egyptian Canadian for Humate Company, Egypt. Amberlite IRA- 400 anion exchange resin for uranium extraction was supplied from Rohm& Hass Co., USA. Other chemicals used in this study were from Merck-USA.
Analytical procedures
Major oxides of the collected sample were chemically analyzed using X-ray fluorescence technique (XRF), Model Rigaku EDXRF spectrometer NEX CG. Also, UV–VIS spectrphotometer (Shimadzu UV-160A) was used for quantitative analysis of REE using 0.015% Arsenazo (III) and Ce as reference according to Marczenko [61]. Uranium was analyzed by an oxidimetric titration method against ammonium metavanadate in the presence of diphenyl amine-4-sulfonic acid sodium salt as indicator [62], while Cu, Zn, Ni, Cr, V …etc. was measured using a Unicam Atomic Absorption Spectrophotometer model-969 (AAS) flame type at proper wavelengths. On the other hand, scanning electron microscope (SEM-EXL 30 Philips type) coupled with X- ray analyzer (EDX unit system) was used for conducting semi- quantitative analysis of the prepared product of U.
Optimization of U leaching process
The leaching process of the concerned spent residue was carried out via pug leaching technique. Using 10 g with size -100 mesh or each round to detect the S/L ratio, time of agitation and concentration of the added reagent. Humic acid was used in this study at different (w/v) ratio. The matrix was pugged for different periods of time (3–21 days). After that the pugged mass was directed to water agitation leaching using different amount of water. Agitation time at different temperature was also studied for removing the dissolved metal ions namely; U(VI), Fe(III), Cu(II) and Zn(II) from the pugged mass. Concentrations of the metal ions were estimated and their leaching efficiencies were calculated. After that, the leaching optimum conditions were investigated, a sample weight of 1 kg was used for the humate leach liquor preparation required for the subsequent U extraction process.
Optimization of U extraction process
The anion exchange resin Amberlite IRA-400 was used for optimizing uranium extraction from the produced humate leach liquor. For this purpose, batch experiments were conducted using different reagent volumes (mL) of wet settled resin (W.S.R) and leach liquor (R/L) at different pH values with stirring for varied period of time (h). The obtained raffinate solutions (V) were analyzed for the U concentration (mgL−1) and extraction efficiency (%) was calculated. The loaded resin, after washing with distilled water, was then subjected for elution process to desorb the loaded uranium using 1 N NaCl solution acidified with 0.2 N H2SO4. The eluate rich uranium solution was directed to U precipitation process using H2O2.
Results and discussion
Chemical composition of the working sample
Chemical composition of the working sample analyses (Table 1) shows different concentration of element constituents, the SiO2, Al2O3 and Fe2O3 considered as the main components representing in the sample by 56.91%, 8.95%, and 8.80% respectively. On the other hand, U, Cu and Zn were found to assay 185 ppm, 2800 ppm and 820 ppm respectively. It is worthy to mention herein that, the high content of U (185 ppm) in this spent residue reflects not only the high radioactive type of waste but also the poor recovering of the prior treatments. A matter which received special interest to be managed and disposed of safety.
Humic acid leaching process
As mentioned above, from the chemical composition and the nature of the studied spent ore residue, it was found necessary to apply the non-conventional humitization leaching process. Several relevant factors have been studied as follow:
Effect of humic acid concentration
In order to study the effect of humic acid concentration upon the leaching efficiencies of the metals of interest. The symmetrically quaternized sampling material was firstly mixed and pugged with humic acid at liquid/solid ratio (L/S) of 1/1 for 3 days at room temperature (25 ± 5 °C) at concentrations ranged between 5 and 15%. The obtained matrix was undergoing water leaching for 30 min. with water/pugged cake (W/PC) ratio of 1/2 at 70 °C. After filtration and washing, the metals of interest were determined, and the dissolution efficiencies were calculated by the mass balance equation.
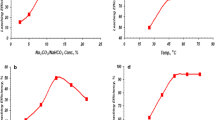
The obtained data (Fig. 1a) showed that, the leaching efficiencies of U, Cu and Zn increased significantly from 19.8, 18.2 and 11.5% to 44.5, 33.1 and 29.3% respectively by increasing the acid concentration from 5 to 13%. On the other hand, the higher increasing in acid concentration than these ratios showed no considerable effect upon the leaching efficiencies of all metal values. This may be attributed to high stability of Cu and Zn complexes with humic acid [63, 64]. With respect to the tendency of Fe, it was found that, the leaching efficiency was relatively low (4.39%) with different HA concentration due to the possibility of reduction to insoluble form of Fe (II) causing precipitation during complex formation with humic acid [65, 66].
Effect of humic/spent ore residue (L/S) ratio
Different ratios of humic acid to spent ore residue ratio (v/w) (L/S) was detected for the dissolution efficiency. These ratios ranged from 1/1 to 3/1 using 13% humic acid, for 3 days pugged time at room temperature. The obtained matrix was then subjected to water leaching for 30 min, 1/2 of water/ pugged cake (W/PC) ratio at 70 °C. The obtained data (Fig. 1b) indicated that, the leaching efficiencies of the metals of interest increased by increasing ratios from 1/1 to 1.5/1, while the pH of the solution kept to 5.8. On the other hand, increasing in humic acid ratio showed a negligible significant effect upon the leachability of U, Cu and Zn. This may be due to the fact that complexes of these elements are very sensitive toward the changes in the pH and hence precipitation process takes place above pH 7. In this context, Pandey et al. [67] reported that, the complex formation at pH 3.50, 1 mol of humic acid complexed with 1 mol of Cu and Fe, whereas in the case of Zn, 1 mol of humic acid complexed with 2 mols of metal. This means that increasing in humic acid ratio effect on pH value but also plays a great role in the formation of complexes and hence the ratio of humic acid (1.5/1) is enough to form complexation with the metals under study.
Effect of pugging time
Time is considered as one of the most effected parameters in the dissolution of ore constituents. This effect was studied at varied time ranged from 3 to 21 days using HA concentration of 13% and (L/S) ratio of 1.5/1 at room temperature. The pugged matrix was then subjected to water leaching for 30 min, and 1/2 water/ pugged cake (W/PC) ratio at 70 °C. From the obtained data in Fig. 1c, it was found that, leaching efficiency of U reached to maximum values 86.80% after 15 days and then decreased by increasing time up to 21 days. The availability of metal ions decreases with time, as well as metals which are built into the crystalline network and also the amorphous structure of the mineral parts of soils [68]. At the meantime, the leaching efficiencies of Cu and Zn were slightly increased from 33.1% and 29.3% to 48.1% and 34.5% respectively by increasing time from 3 to 15 days.
Effect of water agitation time
The effect of water agitation leaching time of pugged cake upon leaching efficiencies of the studied metals was detected in the range from 15 to 60 min. The other leaching conditions were fixed at 2/1 W/PC ratio at 70 °C. The corresponding leaching efficiencies (Fig. 2a) indicated that, 45 min represents the optimum time for dissolving U, Cu and Zn with efficiencies of 89.5, 40.3 and 34.9%, respectively.
Effect of water to pugged cake (W/PC) ratio
Different (W/PC) ratio ranging from 2/1to 4/1 was studied at fixed conditions of 45 min agitating time at 70 °C leaching temperature. The corresponding leaching efficiencies (Fig. 2b) proved that, the high U leaching efficiency reached its maximum value of 92.8% at W/PC ratio of 3/1, while Cu, Zn and Fe leaching efficiencies ranged about 41.2, 34.9 and 3.8% respectively.
Effect of water leaching temperature
The effect of water leaching temperature upon dissolution efficiencies of the assigned metals was studied between 50 and 80 °C, while the other leaching parameters are fixed at 45 min. agitation time and 3/1 W/PC ratio. The obtained results were plotted in Fig. 2c emphasized that, 70 °C represents the optimum temperature for dissolving of U. Beyond this temperature, the leaching efficiency of U was decreased to 89.33%, while Cu, Zn and Fe leaching efficiencies attained 45.7, 37.9 and 5.1% respectively at 80 °C. From the foregoing study, it can be concluded that, the maxmium dissolving of U is around 92.8% from hazardous Abu Zeneima spent residue with the following optimum conditions:
Humic acid conc | 13% |
|---|---|
Pugging time | 15 days at ambient temp |
Humic acid/ spent residue | 1.5/1 |
Water leaching time | 45 min |
water ratio/ Pugged cake | 3/1 |
Agitation leaching temperature | 70 °C |
Kinetic of U dissolution process
The schedule of the technological process for the most optimal condition is pug leaching prior to agitation process. This technique considered as the most effective for liberating uranium from ore material to leachate solution. The optimum leaching conditions for dissolution of U was achieved with 150µ particle size, 13% humic acid, 1/1.5 solid/ liquid ratio and 45 min as agitation time in water medium, which exceed than pugged cake by 3 times (with ratio 3/1 of water/pug cake (W/PC)) at 70 °C. Figure 3 shows parallel increasing of uranium leaching performances with temperature and time, from this data, the optimizing leaching was assigned to be 92.8% at 70 °C and after 45 min of leaching time.
Application of leaching kinetic models
The mathematical model of the un-reacted shrinking core, which is the commonly used for describing the heterogeneous reactions (i.e., mineral leaching from ores). The reaction rate, depending on the phase type, i.e., in the solid–liquid phase, the reaction rate may depends on one/ or more of the following types: (a) liquid film diffusion (mass-transfer), (b) solid layer diffusion, and (c) surface or chemical reactions [69]. The liquid-film diffusion resistance is eliminated or minimized by effective stirring.
In order to determine the uranium type of leaching mechanism, the reaction-models were investigated. The obtained results were analyzed by using the following kinetic rate Eqs. (1 and 2) to get the most fitted reaction mechanism. Reaction rate expression controlled by the surface chemical reaction:
where Kc as rate constant (min−1) for chemical reaction.
Reaction rate expression controlled by the diffusion through the ash or product layer:
where Kd is the rate constant (min−1) for diffusion through the product layer.
Figure 4 (a and b) shows the result of plotting 1− (1−x) 1/3 and 1−3 (1− x) 2/3 + 2(1−x) as a function of time at different temperature values, which produce a straight line relations and the produced slope were assgned as the values of K constants.
The kc and kd values computed from Eqs. (1) and (2). The R2 values are the comparison parameters of fitting the experimental data with the theoretical one. The best fit values of R2 with that close to 1.0. The kc values vary in the rage of 0.010 to 0.014 min−1, while the kd values ranged between 0.009 and 0.015 min−1. The R2 values were in the range of 0.992 to 0.996 and 0.913 to 0.943 for kd and kc respectively. Based on this data, it was found that, the convinced predominant dissolution mechanism of U collected from Abu Zienema pilot plant leached by humic acid is a diffusion controlled only.
Calculation of the activation energy
Arrhenius equation was used for calculation of activation energy, by plotting the logarithmic values (of reactions rate constants: Kd) with the reciprocal of (the absolute) leaching temperature (T) as shown in Fig. 5. The activation energy of the reaction can be calculated using the following equation (3): -
where K is a reaction rate constant, recovery (conversion fraction) in min−1; A is the frequency factor, constant min−1; Ea is the apparent activation energy kJ mol−1; Rg is the universal gas constant = 8.314 JK−1 mol−1; T is the reaction temperature K.
From Fig. 4, the activation energy (Ea) was calculated as follow:—Slope = \(\frac{{ - \text{E}_{a} }}{{\text{R}_{g} }}\).
For Reaction rate expression controlled by the diffusion:—Equation, y = -1.2385
The apparent activation energy (Ea) was calculated from the slope of straight line, which equivalent to 10.297 kJmole−1 for diffusion-controlled reaction models. Based on the (Ea) values, the predominant dissolution mechanism of U from studied waste material was the diffusion controlled only. This value is less than that mentioned by Crundwell (2013) [70], who pointed out this activation energy for diffusion-controlled reactions with values less than 20 kJmol−1 and higher than 40 kJmol−1 for chemical controlled reactions.
Results of uranium extraction process
Applying the above mentioned optimum experimental leaching conditions upon 1 kg of spent ore residue yields 3.0 L of humate solution. The pH of the produced leachate solution is around 5.0 and assaying 57 mg L−1 of U as given in Table 2. This solution was subjected to treatment by Amberlite IRA-400 which well known as strong anion resin exchange for U recovery via equilibrium batch technique.
Optimization of uranium loading process
Effect of pH
The pH of pregnant leaching solution has two significant parameters (a) affected on the metal speciation of the dissolved metal ions, and (b) on the total charge of functional groups in the adsorbent materials. So, it determined the type of binding mechanism to be as ionic exchange or chelating properties. The effect of different pH values upon U loading efficiency with R/L ratio of 0.2/50 and stirring time of 20 min. was studied at pH values ranging from 2.0 up to 5.0. Figure 6a illustrated that; the maximum adsorption efficiency of U reached to 54.3% at pH 3. However, further decreasing in pH value has an opposite effect.
Effect of contact time
The loading capacity was performed at different contact time (i.e., 10, 20, 30, 40 and 50 min.). Under the experimental condition of R/L ratio adjusted to 0.2/50 and pH 3. The obtained data (Fig. 6b) indicated that, the U adsorption efficiency increased from 23.3 to 79.2% by increasing the contact time from 10 to 40 min., this is indicating that the loading performances was relatively high. However, increasing the time more than 40 min. cause a decreasing in the U loading efficiency to 43.7%.
Effect of Resin/Liquid (R/L) ratio
The adsorption efficiency of U was studied at different sorbent dosage, this is by using different ratios of Resin/Liquid (R/L), which ranged from 0.2/50 to 0.8/50 at the optimum experimental condition of pH 3 and at 40 min of contact. The obtained data in Fig. (6c) clarified that, U adsorption efficiency increased with increasing the resin volumes and reached to the maximum value (93.5%) at R/L ratios of 0.8/50. In this context, it is important to mention herein that, the lower extraction efficiency of U may be attributed to competition of some interfering anions e.g. SO42−and Cl− which contest the U upon the resin sites [71].
Results of elution process and uranium precipitation
There are various advantages for using elution process and choice the eluents. It is not only for desorption the loaded ions from resin to use for another cycles, but also to obtain U rich eluate solution suitable for U precipitation. The saturated loaded resin was applied for elution (using 1 N NaCl solution acidified with 0.2 N H2SO4 in batch technique for 40 min stirring in batch technique), after washed with suitable volume of distilled H2O to remove any impurities. Uranium concentration in the eluate solution (assaying 3.4 g/L) was estimated (93.5% elution efficiency %). The expected elution mechanism was appeared in the below equation (4).
Finally, the obtained U rich eluate solution at pH 2.0 was treated with 5% NaOH solution for increasing the pH value to 2.5 and then treated with H2O2 solution to precipitate U. About 99% of U content was precipitated as UO4.2H2O after agitated time of 4 h at room temperature. After filtration and washing, the precipitated uranyl peroxide cake was dried and identified using EDX analysis method as shown in Fig. 7, while the purity was already estimated as 97.3% via chemical analysis method.
Conclusions
Selective leaching was achieved by humic acid toward the carbonaceous shale spent ore residue of Abu Zeneima pilot plant, Southwestern Sinai, Egypt. The residual materials (the studied case) still have high level metal concentration that must be valorized. Among of these ions Ti, Al and Fe with concentrations of 0.53, 8.95 and 8.8% respectively, while other valuable trace elements were also detected in high levels as U, Cu, Zn, REE, V, Co and Cr with concentration of 185, 2800, 820, 70, 58, 125 and 74 mgL−1 respectively. Humic acid used as type of selective leaching agent for dissolving uranium. Based on the concentration values, it can be inferred that the predominant dissolution mechanism of U is diffusion controlled only. Humate leach liquor was prepared by applying optimum pug leaching conditions of 13% humic acid concentration at 70 °C with 45 min stirring time. Amberlite IRA-400 anion exchange resin in batch technique was applied for extraction of about 93.5% of U content at pH 3.0 with high loading kinetic (maximum loading time was achieved by stirring time 40 min) and R/L ratio 0.8/50. Uranyl peroxide was finally obtained (through precipitation from eluate solution) with purity of 97.3%.
References
Tawn EJ, Rees GS, Leith C, Winther JF, Curwen GB, Stovall M, Olsen JH, Rechnitzer C, Schroeder H, Guldberg P (2011) Germline minisatellite mutations in survivors of childhood and young adult cancer treated with radiation. Int J Radiat Biol 87(3):330–340. https://doi.org/10.3109/09553002.2011.530338
Meng F, Yuan G, Larson SL, Ballard JH, Waggoner CA, Arslan Z, Han FX (2017) Removing uranium (VI) from aqueous solution with insoluble humic acid derived from leonardite. J Environ Radioact 180:1–8. https://doi.org/10.1016/j.jenvrad.2017.09.019
Chianese S, Fenti A, Iovino P, Musmarra D, Salvestrini S (2020) Sorption of organic pollutants by humic acids: a review. Molecules 25(4):918. https://doi.org/10.3390/molecules25040918
Ni’am AC, Wang Y-F, Chen S-W, Chang G-M, You S-J (2020) Simultaneous recovery of rare earth elements from waste permanent magnets (WPMs) leach liquor by solvent extraction and hollow fiber supported liquid membrane. Chem Eng Process Process Intensif 148:107831. https://doi.org/10.1016/j.cep.2020.107831
Pavon S, Fortuny A, Coll MT, Sastre AM (2019) Solvent extraction modeling of Ce/Eu/Y from chloride media using D2EHPA. AIChE J 65(8):e16627. https://doi.org/10.1002/aic.16627
Pavon S, Fortuny A, Coll MT, Sastre AM (2018) Neodymium recovery from NdFeB magnet wastes using Primene 81R.Cyanex 572 IL by solvent extraction. J Environ Manag 222:359–367. https://doi.org/10.1016/j.jenvman.2018.05.054
Xia Y, Xiao L, Tian J, Li Z, Zeng L (2015) Recovery of rare earths from acid leach solutions of spent nickel-metal hydride batteries using solvent extraction. J Rare Earths 33(12):1348–1354. https://doi.org/10.1016/s1002-0721(14)60568
Jorjani E, Shahbazi M (2016) The production of rare earth elements group via tributyl phosphate extraction and precipitation stripping using oxalic acid. Arab J Chem 9:S1532–S1539. https://doi.org/10.1016/j.arabjc.2012.04.002
Sinha S, Abhilash MP, Pandey BD (2016) Metallurgical processes for the recovery and recycling of lanthanum from various resources-a review. Hydrometallurgy 160:47–59. https://doi.org/10.1016/j.hydromet.2015.12.004
Abisheva ZS, Karshigina ZB, Bochevskaya YG, Akcil A, Sargelova EA, Kvyatkovskaya MN, Silachyov IY (2017) Recovery of rare earth metals as critical raw materials from phosphorus slag of long-term storage. Hydrometallurgy 173:271–282. https://doi.org/10.1016/j.hydromet.2017.08.022
Hidayah NN, Abidin SZ (2018) The evolution of mineral processing in extraction of rare earth elements using liquid-liquid extraction: a review. Miner Eng 121:146–157. https://doi.org/10.1016/j.mineng.2018.03.018
Wu S, Wang L, Zhang P, El-Shall H, Moudgil B, Huang X, Zhao L, Zhang L, Feng Z (2018) Simultaneous recovery of rare earths and uranium from wet process phosphoric acid using solvent extraction with D2EHPA. Hydrometallurgy 175:109–116. https://doi.org/10.1016/j.hydromet.2017.10.025
Hamza MF, Fouda A, Elwakeel KZ, Wei Y, Guibal E, Hamad NA (2021) Phosphorylation of Guar Gum/Magnetite/Chitosan nanocomposites for uranium (VI) sorption and antibacterial applications. Molecules 26(7):1920. https://doi.org/10.3390/molecules26071920
Hamza MF, Hamad NA, Hamad DM, Khalafalla MS, Abdel-Rahman AA-H, Zeid IF, Wei Y, Hessien MM, Fouda A, Salem WM (2021) Synthesis of eco-friendly biopolymer, alginate-chitosan composite to adsorb the heavy metals, Cd (II) and Pb (II) from contaminated effluents. Materials 14(9):2189. https://doi.org/10.3390/ma14092189
Hamza MF, Gamal A, Hussein G, Nagar MS, Abdel-Rahman AAH, Wei Y, Guibal E (2019) Uranium (VI) and zirconium (IV) sorption on magnetic chitosan derivatives–effect of different functional groups on separation properties. J Chem Technol Biotechnol 94(12):3866–3882. https://doi.org/10.1002/jctb.6185
Hamza MF, Salih KA, Adel A-H, Zayed YE, Wei Y, Liang J, Guibal E (2021) Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem Eng J 403:126399. https://doi.org/10.1016/j.cej.2020.126399
Wei Y, Salih KA, Lu S, Hamza MF, Fujita T, Vincent T, Guibal E (2019) Amidoxime functionalization of algal/polyethyleneimine beads for the sorption of Sr (II) from aqueous solutions. Molecules 24(21):3893. https://doi.org/10.3390/molecules24213893
Wu HY, Chi FT, Zhang S, Wen J, Xiong J, Hu S (2019) Control of pore chemistry in metal-organic frameworks for selective uranium extraction from seawater. Microporous Mesoporous Mater 288:109567. https://doi.org/10.1016/j.micromeso.2019.109567
Dousti Z, Dolatyari L, Yaftian MR, Rostamnia S (2019) Adsorption of Eu(III), Th(IV), and U(VI) by mesoporous solid materials bearing sulfonic acid and sulfamic acid functionalities. Sep Sci Technol 54(16):2609–2624. https://doi.org/10.1080/01496395.2018.1548483
Taha MH (2020) Solid-liquid extraction of uranium from industrial phosphoric acid using macroporous cation exchange resins: MTC1600H, MTS9500, and MTS9570. Sep Sci Technol 17:1562–1578. https://doi.org/10.1080/01496395.2020.1787446
Ahmad M, Yang K, Li L, Fan Y, Shah T, Zhang Q, Zhang B (2020) Modified tubular carbon nanofibers for adsorption of uranium(VI) from water. ACS Appl Nano Mater 3(7):6394–6405. https://doi.org/10.1021/acsanm.0c00837
Riegel M, Schlitt V (2017) Sorption dynamics of uranium onto anion exchangers. Water. https://doi.org/10.3390/w9040268
Masoud AM (2020) Sorption behavior of uranium from sulfate media using purolite A400 as a strong base anion exchange resin. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1763974
Hamza MF, Mubark AE, Wei Y, Vincent T, Guibal E (2020) Quaternization of composite algal/PEI beads for enhanced uranium sorption-application to ore acidic leachate. Gels 6(2):12. https://doi.org/10.3390/gels6020012
Hamza MF, Wei Y, Guibal E (2020) Quaternization of algal/PEI beads (a new sorbent): characterization and application to scandium sorption from aqueous solutions. Chem Eng J 383:123210. https://doi.org/10.1016/j.cej.2019.123210
Hamza MF, Sallam OR, Khalafalla MS, Abd Elhadi AA, Wei Y (2020) Geological and radioactivity studies accompanied by uranium recovery: Um Bogma Formation, southwestern Sinai. Egypt J Radioanalytic Nuclear Chem 324(3):1039–1051. https://doi.org/10.1007/s10967-020-07149-x
Ang KL, Li D, Nikoloski AN (2018) The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 2. Chelat Resins Minerals Eng 123:8–15. https://doi.org/10.1016/j.mineng.2018.04.017
Guo X, Feng Y, Ma L, Gao D, Jing J, Yu J, Sun H, Gong H, Zhang Y (2017) Phosphoryl functionalized mesoporous silica for uranium adsorption. Appl Surf Sci 402:53–60. https://doi.org/10.1016/j.apsusc.2017.01.050
Sarafraz H, Minuchehr A, Alahyarizadeh G, Rahimi Z (2017) Synthesis of enhanced phosphonic functional groups mesoporous silica for uranium selective adsorption from aqueous solutions. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-11993-5
Xu MY, Han XL, Wang T, Li SH, Hua DB (2018) Conjugated microporous polymers bearing phosphonate ligands as an efficient sorbent for potential uranium extraction from high- level liquid wastes. J Mater Chem A 6(28):13894–13900. https://doi.org/10.1039/c8ta02875c
Duval CE, Hardy WA, Pellizzeri S, DeVol TA, Husson SM (2019) Phosphonic acid and alkyl phosphate-derivatized resins for the simultaneous concentration and detection of uranium in environmental waters. React Funct Polym 137:133–139. https://doi.org/10.1016/j.reactfunctpolym.2019.01.015
Yang PP, Liu Q, Liu JY, Chen RR, Li RM, Bai XF, Wang J (2019) Highly efficient immobilization of uranium(VI) from aqueous solution by phosphonate-functionalized dendritic fibrous nanosilica (DFNS). J Hazard Mater 363:248–257. https://doi.org/10.1016/j.jhazmat.2018.09.062
Yuan DZ, Zhang SA, Tan JL, Dai YH, Wang Y, He Y, Liu Y, Zhao XH, Zhang MM, Zhang QH (2020) Highly efficacious entrapment of Th (IV) and U (VI) from rare earth elements in concentrated nitric acid solution using a phosphonic acid functionalized porous organic polymer adsorbent. Sep Purif Technol 237:116379. https://doi.org/10.1016/j.seppur.2019.116379
Horwitz EP, Chiarizia R, Diamond H, Gatrone RC, Alexandratos SD, Trochimczuk AQ, Crick DW (1993) Uptake of metal-ions by a new chelating ion-exchange resin 1 Acid dependencies of actinide ions. Solvent Extr Ion Exch 11(5):943–966. https://doi.org/10.1080/07366299308918195
Sabharwal KN, Nandy KK, Srinivasan TG, Rao PRV (1996) Recovery of uranium from acid media by macroporous bifunctional phosphinic acid resin. Solvent Extr Ion Exch 14(6):1101–1114. https://doi.org/10.1080/07366299608918384
Sabharwal KN, Rao PRV, Srinivasan M (1995) Extraction of uranium by macroporous bifunctional phosphinic acid resin. Solvent Extr Ion Exch 13(3):561–574. https://doi.org/10.1080/07366299508918291
Bhanushali RD, Pius IC, Chetty KV, Vaidya VN, Venugopal V, Rao PRV (2005) Sorption of Pu(IV) on macroporous bifunctional phosphinic acid resin from uranium analytical waste solution. J Radioanal Nucl Chem 265(3):389–394. https://doi.org/10.1007/s10967-005-0838-y
Venkatesan KA, Patre DK, Sabharwal KN, Srinivasan TG, Rao PRV (2000) Kinetics of uranium extraction by macroporous bifunctional phosphinic acid resin. Solvent Extr Ion Exch 18(3):551–565. https://doi.org/10.1080/07366290008934697
Piechowicz M, Abney CW, Zhou X, Thacker NC, Li Z, Lin W (2016) Design, synthesis, and characterization of a bifunctional chelator with ultrahigh capacity for uranium uptake from seawater simulant. Ind Eng Chem Res 55(15):4170–4178. https://doi.org/10.1021/acs.iecr.5b03304
Wei YQ, Qian J, Huang L, Hua DB (2015) Bifunctional polymeric microspheres for efficient uranium sorption from aqueous solution: synergistic interaction of positive charge and amidoxime group. RSC Adv 5(79):64286–64292. https://doi.org/10.1039/c5ra11578g
Alexandratos SD, Zhu X (2016) Polymer-supported aminomethylphosphinate as a ligand with a high affinity for U(VI) from phosphoric acid solutions: combining variables to optimize ligand-ion communication. Solvent Extr Ion Exch 34(3):290–295. https://doi.org/10.1080/07366299.2016.1169148
Ragnarsdottir K, Charlet L (2000) Uranium behaviour in natural environments. Environmental mineralogy: microbial interactions, anthropogenic influences, contaminated land and waste management. Mineralogical Society, pp 245–289
Karlsson S, Sjöberg V, Allard B (2015) Impact of humic substances on uranium mobility in soil–a case study from the Gessenwiese test field, Germany. Uranium-Past and future challenges. Springer, Cham, pp 239–248
Jones D, Dennis P, Owen A, Van Hees P (2003) Organic acid behavior in soils–misconceptions and knowledge gaps. Plant Soil 248(1):31–41
Wu H, Hu Y, Li S (2001) A review on interactions at the interface between organic acids and minerals. Acta Petrologica et Mineralogica 20:399–404
Choppin GR (1988) Humics and radionuclide migration. Radiochim Acta 44(1):23–28
Boguta P, Sokolowska Z (2012) Influence of phosphate ions on buffer capacity of soil humic acids. Int Agrophys 26(1):7–14. https://doi.org/10.2478/v10247-012-0002-1
Sheng G-P, Zhang M-L, Yu H-Q (2009) Quantification of the interactions between a cationic dye and humic substances in aqueous solutions. J Colloid Interface Sci 331(1):15–20. https://doi.org/10.1016/j.jcis.2008.11.006
Seijo M, Ulrich S, Filella M, Buffle J, Stoll S (2009) Modeling the adsorption and coagulation of fulvic acids on colloids by Brownian dynamics simulations. Environ Sci Technol 43(19):7265–7269. https://doi.org/10.1021/es9002394
Liao J, Wen W, Li B, Yang Y, Zhang D, Kang H, Yang Y, Jin J, Liu N (2013) Interaction between uranium and humic acid (II): complexation, precipitation and migration behavior of U (VI) in the presence of humic substances. Nuclear Sci Techniques 24(3):30301. https://doi.org/10.13538/j.1001-8042/nst.2013.03.010
de Melo BAG, Motta FL, Santana MHA (2016) Humic acids: structural properties and multiple functionalities for novel technological developments. Mater Sci Eng 62:967–974
Khan S, Khan NN (1986) The mobility of some organophosphorus pesticides in soils as affected by some soil parameters. Soil Sci 142(4):214–222
Gebremariam SY, Beutel MW, Yonge DR, Flury M, Harsh JB (2012) Adsorption and desorption of chlorpyrifos to soils and sediments. Reviews of environmental contamination and toxicology, Springer, New York, pp 123–175
Amer T, EL-Hazek M, Abd EL-Fattah N, EL-Shamy A, Abd-Ella M, EL-Shahat M (2010) Processing of Abu Zenima mineralized gibbsite ore material for the recovery of aluminium, zinc and individual light rare earth oxides. J Middle East Radioact Isotopes Center 40(2):1–11
Abdellah W, Amer T, Abdel Wahab G, AlShami A, El-Shahat M (2014) Extraction of boron and vanadium from Abu Hamata alkali leach solution by using ion exchange resin. Int J Eng Res 3(3):2033–2038
Wahab SA, Rezik A, Khoziem HAA, Khalid E, Abdellah W (2019) Kinetics of uranium carbonate leaching process from carbonaceous shale, southwestern Sinai. Egypt Euro-Mediterr J Environ Integr 4(1):1–11
Abu Khoziem HA (2017) Recovery of Cu, REEs, U and V from Abu Zienema poly-mineralized carbonaceous shale ore material, southwestern Sinai. Egypt Bulletin Fac Sci Zagazig Univ 2017:114–133
Abu Khoziem HA, Khalafalla MS, Abdellah WM (2021) Green Recovery of Uranium from Abu Zienema Mineralized Carbonaceous Shale, West Central Sinai, Egypt. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1983556
Hamza MF, El Aassy IE (2014) Solid phase extraction of uranium removal from underground water, Wadi Naseib, Southwestern Sinai. Egypt Desalination Water Treat 52(1–3):331–338. https://doi.org/10.1080/19443994.2013.794584
Hamza MF (2015) Removal of uranium (VI) from liquid waste of calcareous shale, Allouga, southwestern Sinai. Egypt Desalination Water Treat 54(9):2530–2540. https://doi.org/10.1080/19443994.2014.900650
Marczenko Z (1975) Spectrophotometric determination of elements. E Horwood, pp 20–43
Bickel M (1997) The Davies-Gray titration for the assay of uranium in nuclear materials: a performance study. J Nucl Mater 246(1):30–36. https://doi.org/10.1016/S0022-3115(97)00040-8
Plaza C, Senesi N, García-Gil J, Polo A (2005) Copper (II) complexation by humic and fulvic acids from pig slurry and amended and non-amended soils. Chemosphere 61(5):711–716. https://doi.org/10.1016/j.chemosphere.2005.03.046
Plaza C, D’orazio V, Senesi N (2005) Copper (II) complexation of humic acids from the first generation of EUROSOILS by total luminescence spectroscopy. Geoderma 125(1–2):177–186. https://doi.org/10.1016/j.geoderma.2004.07.012
Emmenegger L, Schönenberger R, Sigg L, Sulzberger B (2001) Light-induced redox cycling of iron in circumneutral lakes. Limnol Oceanogr 46(1):49–61. https://doi.org/10.4319/lo.2001.46.1.0049
Lippold H, Evans ND, Warwick P, Kupsch H (2007) Competitive effect of iron (III) on metal complexation by humic substances: characterisation of ageing processes. Chemosphere 67(5):1050–1056. https://doi.org/10.1016/j.chemosphere.2006.10.045
Pandey AK, Pandey SD, Misra V (2000) Stability constants of metal–humic acid complexes and its role in environmental detoxification. Ecotoxicol Environ Saf 47(2):195–200. https://doi.org/10.1006/eesa.2000.1947
Bohn H, McNeal B, O’Connor GAO (1979) Soil Chemistry. Wiley, New York
Levenspiel O (1999) Chemical reaction engineering. Wiley, New York
Crundwell F (2013) The dissolution and leaching of minerals: Mechanisms, myths and misunderstandings. Hydrometallurgy 139:132–148. https://doi.org/10.1016/j.hydromet.2013.08.003
Morais CA, Ladeira A (2008) The influence of competitive species on uranium recovery using resin and solvent extraction techniques In: Proceedings of the Sixth International Symposium of Hydrometallurgy Society for Mining, Metallurgy & Exploration, Inc., Littleton, CO 292–296
Acknowledgements
This work was supported by the Egyptian Nuclear Materials Authority. Special dedication is given to memory of Prof. Dr. Omneya El Hussaini.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalafalla, M.S. Biotechnological recovery of uranium (VI) from Abu Zeneima spent ore residue using green lixiviant. J Radioanal Nucl Chem 331, 2503–2513 (2022). https://doi.org/10.1007/s10967-022-08249-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08249-6