Abstract
A solid waste sample from acidic leaching of Alluoga sedimentary rocks, SW Sinai, Egypt, was processed for leaching and extraction of U and Cu respectively. This sample assayed 200 mg/kg U and 5 g/kg Cu. The present work aims to obtain solid waste free of U and Cu through selective alkaline leaching for uranium followed by environmentally safe glycine solution for copper leaching from the resulted waste. Under studied optimum conditions the leaching efficiency of U and Cu attained 93% and 96% respectively. Uranium was extracted using Amberlite IRA-400 Anion exchanger while Cu was selective extracted by LIX-973N diluted in kerosene. The relevant factors affecting the Cu extraction process were adequately studied and the number of stages for extraction and stripping were determined by the construction of McCabe–Thiele diagram.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uranium is the main fuel element in many commercial nuclear reactors [1, 2]. Different lixiviant were used as leaching agent for copper and uranium in ores. Sulphuric acid is the most widely used due to cheap cost, however it is not economic for ores containing gangue minerals such as calcite, dolomite leading to consume huge amount of acids and the process become uneconomical [3]. Alkaline leaching of uranium using sodium carbonate and sodium bicarbonate systems used almost exclusively for commercial uranium recovery, where the soluble and stable uranyl tricarbonate [UO2(CO3)3]4− ion is formed [4, 5]. On the other hand, it is very selective in nature because the most of metals are not soluble in alkaline solutions and the obtained leach liquor is relatively pure, easy recycling in addition to minor corrosion problems [5,6,7]. The time of leaching in carbonate leach liquor varies from several to more than 24 h which can be greatly decrease by increasing temperature of process [8]. Several techniques have been used to recover U from its leach liquor prepared by leaching processes of its ore materials such as direct precipitation, ion exchange resin and organic solvents. The choice of suitable method depends on many factors such as the leaching medium, the concentration of uranium in the leach liquor, the amount and concentration of the co dissolved impurities as well as upon the desired final U product purity. The direct precipitation and solvent extraction techniques are favorable for U recovery from high concentrated solutions (higher than 900 mg/L) whereas ion exchange used for pre-concentration prior to precipitation applied on leach liquor have low concentration of U range below 350 mg/L [9]. In alkaline leach liquor, the presence of anionic species mainly as uranyl carbonate complexes, [UO2 (CO3)2]2− and [UO2 (CO3)3]4− [7] lead to use of anion exchanger. Several authors have been studied the alkaline leaching of uranium and its recovery from alkaline leach liquor [7, 10,11,12].
The production of about 80% of Cu from their ores is obtained by floatation, smelting, and refining, while the other 20% is obtained by hydrometallurgical processes through leaching, solvent extraction and electrowinning [13]. Several authors studied the leaching behavior of copper ores using nonconventional reagent organic acids such as citric acid [14] and adipic acid [15].
Glycine is the smallest amino acid has chemical formula NH2CH2COOH, it is nonpolar and characterized by the presence of an amine and carboxylic group bonded to the same carbon atom in α position make it act as an acid (proton donor) and/or a base (proton acceptor) which make it to have amphoteric nature depending on pH when dissolved in water. The three different forms in an aqueous solution namely the cationic at below pH of 2.35, anionic above pH 9.78 and zwitterion between pH 2.35 and 9.78 is shown in the following Eq. (1) [16].
Glycine is predominantly in the zwitterionic form between pH 2.35 and 9.78, the dissociation of zwitterionic glycine according to Eq. (2), takes place as an intermediate step even if the zwitterion (HL) is dominant and subsequent complexation of CuO(s) will take place, according to Eq. (3)
Different soluble complexes formed between glycine and Cu is shown in the following equations with their stability constants [16].
According to Eq. (2), as pH increases the dissociation of HL into the anionic form (L−) will increase and subsequently increases the formation of CuL2 by Eq. (3) which has the highest stability constant of 15.64 relative to the other copper-glycine complexes [17].
Glycine act as chelating ligand, so it has ability to form complexes with metals such as Cu, Co, Ag and Au through oxygen atom of carboxylic group and nitrogen atom of amine group [18, 19]. Oraby and Eksteen stated that glycine has selectivity toward copper over other metals such as iron and lead [20]. Different authors have investigated the leaching of Cu from different types of copper-bearing minerals [20,21,22,23,24,25,26] and from waste printed circuit boards [27] using alkaline glycine solution.
The main advantages of non-conventional leaching using glycine over the conventional other leaching agents are non-toxic, environmentally safe, chemically and thermally stable over a wide pH and Eh range, in addition to its low cost and large scale production [17], it is used in animal feed in addition to pharmaceutical industries, for that it is commercially available [20].
Solvent extraction is one of the most important methods that used for the separation of metal ions from leach liquor [28]. From previous studies stated by Feizollahi and Azizi which illustrated that there are two classes of main extractants to extract Cu from leach liquor, the first is chelating extractants including ketoximes and aldoximes the second is acidic and solvating extractants [13]. Oximes and β-diketone extractants have been extensively studied and applied commercially to recover Cu from acidic and alkaline aqueous solutions [29,30,31]. Eksteen et al. [17] and Tanda et al. [31] illustrated that Cu can be recovered from aqueous copper-glycinate complexes using solvent extraction.
The objective of this study is to obtain selective leaching of uranium from solid waste sample using alkaline reagents, followed by leaching of copper from the resulted waste. The former is recovered using anion exchange while the latter using solvent extraction technique.
Experimental
Characterization of solid waste sample and analytical technique
The solid waste sample was collected from the remaining waste after acid processing of Alluoga sedimentary rocks south western Sinai, Egypt using sulphuric acid.
Analytical techniques and instrumental analyses
Chemical analyses
The solid waste sample was dried, grinded (≈ 75 μm) sizes and then subjected to complete chemical analyses for its major constituents using the rapid silicate analytical procedure [32], the loss on ignition (L.O.I.) was determined gravimetrically at 110 °C and 550 °C for H2O corresponding to humidity and organic matter respectively as well as at 1000 °C for CO2.
Uranium was analyzed using the oxidimetric titration method against ammonium metavanadate which was used after its reduction using ammonium ferrous sulfate [33].
Instrumental analyses
-
a.
X-ray fluorescence technique (XRF)
The trace elements were analyzed by the X-ray fluorescence technique (XRF), Philips Unique II unit fitted with an automatic sample changer PW 1510 (30 position), which was connected to a computer system using X-40 program for spectrometry, in the Nuclear Materials Authority Laboratories (NMA Lab).
-
b.
Flame atomic absorption (FAAS)
Copper was analyzed during experiments using the flame atomic absorption, (FAAS), Unicam 969 of (NMA Lab).
-
c.
Environmental Scanning Electron Microscope (ESEM)
Finally, the obtained products were analyzed using Philips model XL30 supported by energy depressive X-ray unit (EDX) of (NMA Lab).
Recovery procedures
Recovery of uranium
Selective alkaline leaching process for uranium
The leaching of uranium was applied upon solid waste sample using carbonate system. To optimize the relevant leaching factors several leaching experiments have been performed by agitating a suitable weight of the residual sample with a suitable volume of alkaline reagent of specified concentration at certain liquid/solid ratio for certain period of time at the required temperature using stirring speeds about (450 rpm). After each experiment the treated slurry was filtered and the residue left behind washed with distilled water, both of filtrate and washings were made up to volume before analysis.
Uranium extraction
Applying the optimum leaching conditions upon about 1.5 kg of the solid waste sample, after filtration and washing yielded 5 L of carbonate leach liquor. U was selectively recovered through the anion exchanger Amberlite IRA400, followed by elution from the anion exchanger. Then, U can be precipitated from collected eluate samples.
Recovery of copper
Leaching process for copper using glycine solution
The second waste after alkaline leaching of uranium which contain insoluble copper element, was dried and then reused in leaching of copper using glycine solution, to obtain the optimum copper leaching conditions.
Copper extraction
Applying the optimum copper leaching conditions using glycine solution upon 0.25 kg of second waste, after filtration and washing it yielded 1.2 L which was used for copper recovery using solvent extraction technique. LIX-973 N diluted in kerosene was used to extract Cu. Extraction and stripping experiments were performed in separatory funnels by mixing the prepared organic and the aqueous phase and then shaken for a required time. After phase disengagement, the raffinate was separated and was analyzed by AAS, while that in the organic phase was obtained by a difference. Solvent extraction factors were studied such as solvent concentration, the optimum contact time, the pH of the leach liquor and O/A ratios. The stripping behavior of Cu using different concentrations of sulfuric acid solutions and the relevant factors of contact time and A/O ratios were studied.
Results and discussions
Chemical characteristics of studied sample
There are different radioactive minerals in Allouga area, which were previously recognized by different authors [34, 35] such as pitchblende (Triuranium octoxide, U3O8), brannerite (U,Ca,Ce)(Ti,Fe)2O6), uraninite (UO2), carnotite [K2(UO2)(VO4)nH2O], uranophane [Ca(UO2)2SiO3], sklodovvskite [Mg[(UO2)(SiO3OH)]2(H2O)6] and torbernite (CuU2PO4), in addition to the base metal minerals are chalcopyrite [CuFeS2], chalcocite [Cu2S], malachite [Cu2 CO3 (OH)2] and azurite [Cu3(CO3)2(OH)2].
From the chemical analysis of major oxides and trace elements of the studied solid waste sample which were subjected to complete chemical analyses are shown in Table 1. The results indicate that residue mainly consist of SiO2 (53.45%), Fe2O3 (11.96%) and Al2O3 (7.65%). On the other hand, both of CaO and MgO assayed 4.48% and 3.22% respectively. With respect to the metal values, assays were 5000 mg/kg, 327 mg/kg, 322 mg/kg and 200 mg/kg for Cu, Sr, Cr and U respectively.
Leaching processes
The leaching processes of solid waste sample involve two steps; the first is selective for U leaching using alkaline technique while the second will apply upon second waste resulted from the previous step which is used for Cu leaching using glycine solution.
Uranium alkaline leaching
Effect of Na2CO3/NaHCO3 concentration
The effect of mixed (3:1) Na2CO3/NaHCO3 solution concentration upon U leaching efficiency was studied ranging from 2.5 to 20%, while the other leaching conditions were fixed at 25 °C leaching room temperature for 120 min. leaching time and (3/1) liquid/solid ratio. From the data shown in Fig. 1a U leaching efficiency increases from 15.6 to 50% as the concentration of mixed Na2CO3/NaHCO3 increases from 2.5 to 10%, further increase of alkali mixture concentration up to 20% have no effect in U leaching efficiency. Therefore, 10% alkali mixture concentration represents the preferred concentration for the other experiments with leaching processes.
Effect of liquid to solid ratio
The effect of liquid/ solid ratio ranging from 1/1/ to 5/1 upon U leaching efficiency were studied at the concentration of 10% mixed (3:1) Na2CO3/NaHCO3, 25 °C leaching room temperature for 120 min. leaching time. The obtained results as shown in Fig. 1b indicate that the leaching efficiency increases from 1/1 to 3/1, further increases in liquid / solid ration lead to decrease in U leaching efficiency, which reached to 30.6% at L/S ratio of 5/1 and this may be related to leaching of other interfering elements [7]. Hence L/S ratio of 3/1 was considered as the best condition for other leaching experiments.
Effect of temperature
The effect of reaction temperature upon U leaching efficiency was investigated ranging from 25 °C room temperature up to 85 °C, the other conditions were fixed at 10% mixed (3:1) Na2CO3/ NaHCO3, L/S ratio of 3/1 for 120 min. leaching time. As shown in Fig. 1c the U leaching efficiency increases from 50 to 93% as temperature increases from 25 to 85 °C. Improving the U leachability with increasing temperature might be due to increase in mobility of ions under the effect of given energy; also this indicates that the alkaline leaching is an endothermic process [36]. Thus the preferred leaching temperature for U leaching processes is 85 °C.
Effect of contact time
To study the effect of leaching time upon U leaching efficiency, several experiments were carried out at different time ranging from 60 to 180 min. leaving the other conditions at 10% mixed (3:1) Na2CO3/ NaHCO3, L/S ratio of 3/1 and 85 °C leaching temperature. It is clear from the results obtained which are shown in Fig. 1d as the time increases from 60 to 120 min., the U leaching efficiency increases from 61.2 to 93%. Further increase in leaching time up to 180 min., has no effect in U leaching efficiency. Therefore, 120 min. is the suitable leaching time for U leaching processes.
From the previous results, the optimum leaching conditions for leaching about 93% of U from solid waste sample in Alluoga locality, southwestern Sinai, Egypt are, 10% mixed (3:1) Na2CO3/NaHCO3 at liquid/solid ratio of 3/1 for 120 min. leaching time and 85 °C leaching temperature.
Uranium extraction
A weighted 1.5 kg of Alluoga solid waste was leached using previous optimum alkaline leaching factors, after filtration and washing yielded 5 L of carbonate leach liquor with the total content of uranium being 279 mg/5L. U was recovered using Amberlite IRA 400 anion exchanger.
Uranium adsorption
The pH of leach liquor was firstly adjusted at 8.5 using H2SO4, after that allow leach liquor to pass through resin column of 0.5 cm radius containing about 3.8 ml wet settled resin (w s r). About 100 ml of 5% Na2CO3 passing through resin column for activation. A contact time of 5 min. equivalent to a flowrate of 0.6 ml/min was used [37], the effluent sample was collected every 200 ml and analyzed for uranium, the total adsorbed uranium content on the resin was 220 mg. Referring to the theoretical capacity of Amberlite IRA-400 of 1.56 meq/ml, the U adsorption capacity would amount to 92.8 mg U/ml resin, therefore, the total U adsorbed with adsorption efficiency of 78.9% was obtained. This relatively low efficiency was due to presence of iron which would compete with the uranyl tricarbonate complex UO2(CO3)34− for the available exchange sites.
Uranium elution
In elution process, the resin column was firstly washed with distilled water to displace any trapped solution. Prepared about 50 ml eluant solution using 1 M NaCl with 0.25 M Na2CO3 [12] allow passing through saturated resin using a contact time of 6 min. equivalent to flowrate of 0.5 ml/min. [37]. The elution efficiency of U was 95%. Then the pH of eluted solution was adjusted at 12 using 10% NaOH where U was precipitated as sodium di-uranate (Na2U2O7) according to the following Eq. (7).
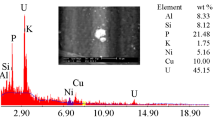
The obtained precipitate was dried at 110 °C and then analyzed by ESEM- EDX Fig. 2. The U assay in the product amounted to 77.92% associated with some impurities such as Na (9.69%), Si (8.94%), K (0.68%) and Fe (2.77%).
Copper leaching
The second waste after previous carbonate leaching processes which contained insoluble copper was dried and was used for studying the attempt of leaching with glycine solution; the concentration of Cu was 5200 mg/kg which result due to decrease in weight of this residue after alkaline leaching of uranium.
Effect of glycine concentration
The effect of glycine concentration on Cu leaching was investigated by using various concentrations of the glycine that ranged from 0.75 to 4.5%, while the other conditions keeping at 120 min. leaching time, liquid /solid ratio of 3/1 and at 25 °C room temperature. The measured pH at 0.75% and 4.5% glycine concentration before leaching processes were 9.5 and 8.5 respectively; hence pH was kept constant at 9.5.The obtained results shown in Fig. 3a indicate that the leaching efficiency of Cu increases from 35.7 to 54% as the glycine concentration increases from 0.75 to 3%, this is due to more glycinate anions are available around minerals surface of Cu which is consistent with [24, 26]. Further increase in glycine concentration has no effect in Cu leaching efficiency. Choice of 3% glycine concentration was the best and leaching efficiency can be improved by variation in the other leaching conditions.
Effect of initial solution pH
To study the effect of initial leach solution pH upon Cu leaching efficiency, the initial pH was varied from 9.5 to 11.5 using NaOH and the other conditions were fixed at 3.0% glycine concentration for 120 min. leaching time, liquid /solid ratio of 3/1 and at 25 °C room temperature. From the results illustrated in Fig. 3b indicate that the best leaching efficiency for Cu attained 54% at pH 9.5, the leaching efficiency decreases by increase in pH to reach 20.4% at pH 11.5. This result is consistent with Oraby and Eksteen [20], Reda and Awny [26] at a certain time. Tanda reported that depending on the temperature, pH and Cu concentration the Cu-glycinate complex has an upper limit of solubility in alkaline solutions and precipitated as Cu(Gly)2 when copper concentration was more than 3.1 g/L at pH 10.2 and 25 °C [24]. On the other hand O'Connor et al. stated that above pH 11, the ability of buffer solution is lost and the equilibrium of Eqs. 8 and 9 are shifting to the right to form both of insoluble CuO and Cu2O which occupy and block active surface sites of Cu waste sample and will slow the dissolution of copper [25].
For that pH 9.5 is the best choice and improving the Cu leaching efficiency by variation in other leaching conditions can be achieved.
Effect of hydrogen peroxide
To study the effect of hydrogen peroxide as an oxidant upon copper leaching efficiency, the initial peroxide concentrations were varied from 0.0 to 2% (v/v) at fixed conditions of 3.0% glycine concentration, pH 9.5 for 120 min. leaching time, liquid /solid ratio of 3/1 and at 25 °C room temperature. The results obtained which are illustrated in Fig. 3c show slight improvement in Cu leaching efficiency reaching to 56.5%, 58.4% and 60% at 0.5%, 1% and 1.5%H2O2 respectively. Further increase in H2O2 concentration had no effects and this was in agreement with [20, 24]. So, choice of 1.5% H2O2 is the best condition.
Effect of liquid to solid ratio
The effect of L/S ratio upon Cu leaching efficiency was studied from 1/1 to 6/1, the other variables were fixed at 3.0% glycine concentration, pH 9.5, 1.5% H2O2, for 120 min. leaching time, liquid /solid ratio of 3/1 and at 25 °C room temperature. The results obtained as shown in Fig. 3d indicate the leaching efficiency of Cu increasing from 39.7 to 69% as L/S ratio was increased from 1/1 to/ 4/1 and further increase in L/S ratio did not affect upon Cu leaching efficiency, therefore L/S ratio of 4/1 was chosen as the best ratio.
Effect of contact time
The effect of leaching time upon Cu leaching efficiency was investigated ranging from 60 to 300 min. The other leaching conditions were fixed at 3.0% glycine concentration, pH 9.5, 1.5% H2O2, liquid /solid ratio of 4/1 and at 25 °C room temperature. The results as shown in Fig. 3e indicate the leaching efficiency of Cu increasing from 55.3 to 78.3% as time increased from 60 to 180 min., on further increase in leaching time, the Cu leaching efficiency was not affected. Leaching time of 180 min. was chosen as optimum condition.
Effect of temperature
Several experiments were done at different temperatures ranging from 25 to 55 °C, the other leaching conditions were fixed at 3.0% glycine concentration, pH 9.5, 1.5% H2O2, for 180 min., liquid /solid ratio of 4/1. From results obtained as illustrated in Fig. 2f the temperature has significant effect in Cu leaching efficiency, where the leaching efficiency of Cu attained 96% at 55 °C compared to 78.5% at 25 °C, which is in agreement with Eksteen, et al. who stated that increase in temperature to 60 °C was highly sensitive upon copper leaching efficiency from chalcopyrite ore [17].
From the previous results, the optimum leaching conditions for leaching about 96% of Cu from second waste after extraction of uranium in Alluoga locality, southwestern Sinai, Egypt is 3.0% glycine concentration, pH 9.5, 1.5% H2O2, for 180 min., liquid/solid ratio of 4/1 and 55 °C leaching temperature.
Copper extraction
Applying the optimum copper leaching conditions upon second residue which is free from uranium upon 0.25 kg and after washing, filtration, the obtained copper concentration is 1041 mg/kg and total content is 1.25 gm/1.2 L with 96% copper leaching efficiency. The acquired leach liquor was subjected to solvent extraction process using LIX-973N diluted in kerosene as extracting agent. To optimize Cu extraction, relevant factors including pH of the aqueous phase, solvent concentration and extraction time were firstly studied.
Copper solvent extraction
Effect of aqueous phase pH The effect of pH upon the recovery of Cu from glycine leach liquor was determined in the range from 9 to 11 by adjusting the pH with NaOH or H2SO4, 5% solvent concentration diluted in kerosene with O/A ratio of 1/1 for 5 min contact time. The results are illustrated in Fig. 4a. The Cu extraction efficiency increases from 75.1 to 99.5% as pH increases from 9 to 10.5, for that pH of 10.5 was chosen to be the best condition. This can be interpreted from Eq. (10).
as it is clear that hydrogen atoms are released during the extraction of copper from the leach solutions, this suggest that an increase in pH should increase the extraction of Cu since this allows the aqueous phase to return to its natural acidity, and this is consistent with Eksteen et al. [17] and Potgieter [38] who stated that increasing the pH of the aqueous phase improved the extraction of copper from glycine leach liquor using LIX-84I as solvent extractant.
Effect of LIX-973N concentration The effect of LIX-973N concentration on Cu extraction efficiency was studied by varying the concentration of extractant from 1 to 7% (v/v) in kerosene at, O/A ratio of 1/1, pH 10.5 and 5 min. contact time. From Fig. 4b, it is clear that Cu extraction efficiency increases from 73.4%, 85.2% and 99.5% as the extractant concentration increases from 1%, 3% and 5%, slight increase reaches to 99.7% at extractant concentration of 7%. This is a result of the equilibrium of the reaction illustrated in Eq. (10), where as concentration of extractant increase will push the equilibrium to the right. Thus, 5% LIX-973N was the best choice for Cu extraction.
Effect of contact time The impact of contact time between the aqueous and organic phases upon Cu extraction efficiency was investigated in the range between 1 and 5 min. at 5% solvent concentration diluted in kerosene at O/A ratio of 1/1 and pH 10.5. The results in Fig. 4c indicate that Cu extraction is very rapid as it reached to 95.6% at first min. and increased to 99.5% at 3 min. Further increase in contact time has no effect in Cu extraction efficiency, for that 3 min. is chosen as optimum contact time. This result agrees with El Hazek et al. [39] who recovered Cu using the same LIX-973N extractant but from sulphate leach liquor.
Effect of organic/aqueous (O/A) ratioThe effect of organic to aqueous phase ratio O/A has been studied in the range from 1/4 up to 4/1on Cu extraction efficiency at 3% solvent concentration diluted in kerosene, at pH 10.5 and 3 min. contact time. From results as shown in Table 2, the variation of O/A ratio from 1/4 to 4/1 led to increase in Cu extraction efficiency from 49.0 to 100.0%.
In order to determine the number of theoretical stages needed to obtain the maximum extraction for Cu, the McCabe–Thiele diagram was constructed by contacting the organic phase with the aqueous solution at different O/A phase ratios varying from 1:4 to 4:1 as shown in Fig. 4d, the varying of O/A ratio would realize Cu loading in organic phase up to 2.55 g/l, and 3 stages would realize depletion in aqueous phase at which Cu concentration equal 0.0
Copper stripping
Cu can be stripped from Cu loaded extactant using strong acid as shown in the following Eq. (11)
In the Cu industry, H2SO4 or HNO3 are widely used to strip Cu from solvents [38]. In the present work economically sulphuric acid was used as stripping reagent.
The loaded organic extractant with Cu had been prepared for stripping processes at conditions of 5% solvent concentration diluted in kerosene with O/A ratio of 1/1, pH 10.5 and 3 min. contact time, the stripping of Cu loaded extractant assaying 1036 mg/L was investigated using H2SO4 solution.
Effect of contact time upon Cu stripping The effect of contact time upon Cu stripping efficiency was studied in the range from 2 to 10 min. at 2 M H2SO4 and A/O ratio of 1/1, the results shown in Fig. 5a indicate that the Cu stripping efficiency increases from 43.6 to 53.6% as time increases from 2 to 8 min., further increase to 10 min. had slightly increased to 54.1% Cu stripping efficiency. Thus 8 min. is the best choice for contact time.
Effect of H2SO4 concentration Different experiments were done to investigate the effect of sulphuric acid concentration upon Cu stripping efficiency in the range between 0.5 and 4 M at 8 min. contact time and A/O ratio of 1/1. It is clear from Fig. 5b that as H2SO4 concentration increases from 0.5 to 2 M, Cu stripping efficiency increases from 41.3 to 53.6%. The slight increase at 4 M H2SO4 reaches Cu efficiency to 57%. This leads to choice of 2 M H2SO4 concentration as the best condition to economize acid consumption.
Effect of aqueous /organic (A/O) ratio The effect of A/O ratio varying from 1/4 to 4/1 upon Cu stripping efficiency was investigated at 2 M H2SO4 concentration and 8 min contact time. The results as shown in Table 3 reveal that Cu stripping efficiency increases from 19.8 to 90.1% by varying A/O from 1/4 to 3/1 and complete stripping was achieved at 4/1 ratio. On the other hand, to determine the number of theoretical stages required for complete stripping of Cu, the McCabe–Thiele diagram was constructed by contacting the aqueous solution with the loaded organic phase at different A/O ratios varying from 1:4 to 4:1 as shown in Fig. 5d, which indicate 3 stripping stages, leaving about 720 mg/l in the stripping solution.
In order to obtain marketable product of Cu, it can be obtained by precipitation from the stripping solution of (CuSO4) by adjusting pH to 5.5 [12] using NaOH according to the following Eq. (12)
The obtained blue copper hydroxide precipitate was washed and dried at 110 °C and was subjected to ESEM- EDX analysis as shown in Fig. 6.The purity is 72.85% Cu and 27.15% S.
Conclusion
Solid waste sample from acidic leaching of different ores in Alluoga locality, SW Sinai, Egypt was subjected to selective alkaline leaching for uranium. Under the best conditions using10% of mixed (3:1) Na2CO3/NaHCO3 at liquid /solid ratio of 3/1, for 120 min. and 85 °C leaching temperature, the U leaching efficiency attained 93%. About 5 L was prepared using 1.5 kg of studied solid waste sample, the total contents of uranium assayed 279 mg/5L. Amberlite IRA 400 anion exchanger was used for U extraction. The second waste after previous carbonate leaching containing insoluble Cu was dried and processed for Cu leaching using glycine solution. The leaching efficiency of Cu was 96% under optimum leaching conditions of 3.0% glycine concentration, at liquid /solid ratio of 4/1, pH 9.5, 1.5% H2O2, for 180 min., and 55 °C leaching temperature. LIX-973N will be used as chelating extractant for copper diluted in kerosene. The extraction efficiency of Cu attained 99.5% at 5% LIX-973N diluted in kerosene, pH 10.5, O/A of 1/1 and contact time of 3 min, while stripping efficiency is 53.6% using 2 M H2SO4 at A/O of 1/1 and contact time of 8 min. The products of U and Cu results from precipitating the former from eluted samples and the latter from stripping sample are subjected to ESEM- EDX analysis. Finally, all the obtained results have been summarized in the following flowsheet as shown in Fig. 7.
References
Sreenivas T, Chakravartty JK (2015) Alkaline processing of uranium ores of Indian origin. Trans Indian Inst Met 69(1):3–14. https://doi.org/10.1007/s12666-015-0548-2
Uranium 2014 (2014) Resources, Production and Demand, in Joint Report by the OECD Nuclear Energy Agency and the IAEA, Vienna, p 508.
You-Cai L, Wei Y, Jian-Gang F, Li-Feng L, Dong Q (2013) Leaching kinetics of copper flotation tailings in aqueous ammonia/ammonium carbonate solution. Can J Chem Eng 91(4):770–775
Khan Y, Shah S S, Siddiq M (2012) Selection of lixiviant system for the alkaline in-situ leaching of uranium from an arkosic type of sandstone and measuring the dissolution behaviour of some metals and non-metals. J Chem Soc Pak 34, No.4
Santos Elizangela A, Ladeira Ana Claudia Q (2009) Leaching of uranium from the Osamu Utsumi mine wastes, INB Caldas, Minas Gerais, Brazil. INAC International Nuclear Atlantic Conference—INAC
El-Ansary AL, Abd El Wahab GM, Bayomi EE, Nouhb EA (2018) Purification of Abu-Zenima wet crude yellow cake using alkaline leaching of U(VI), Egypt. J Pet 23(4):523–530. https://doi.org/10.1016/j.ejpe.2017.08.003
Abdel Wahab S, Rezik A, Abu Khoziem H, Khalid K, Abdellah W (2019) Kinetics of uranium carbonate leaching process from carbonaceous shale, southwestern Sinai, Egypt. Euro-Mediterr J Environ Integr 4:19. https://doi.org/10.1007/s41207-019-0106-0
Frąckiewicz K, Kiegiel K, Herdzik-Koniecko I, Chajduk E, Zakrzewska-Trznadel G, Wołkowicz S, Chwastowska J, Bartosiewicz I (2012) Extraction of uranium from low-grade Polish ores: dictyonemic shales and sandstones. Nukleonika 58(4):451–459
Hamza MF, Sallam OR, Khalafalla MS, Abbas AEA, Wei Y (2020) Geological and radioactivity studies accompanied by uranium recovery: Um Bogma Formation, southwestern Sinai, Egypt. J Radioanalyt Nucl Chem 324:1039–1051. https://doi.org/10.1007/s10967-020-07149-x
Bakry AR, El-Hady SM (2015) Alkaline selective leaching of uranium from carbonate-rich black shale wadi naseib, southwestern Sinai, Egypt. CTAIJ 10(6):266–275
Abu Khoziem HA (2017) Mineralogical characteristics and leachability of some valuable metals from Abu Zienema poly mineralized carbonaceous shale, southwestern Sinai, Egypt. J Sedimentol 23:57–70
Oraby A, El- Skeikh E, Salah W, El- Saied F, El Gendy H, Ismaiel D (2019) Recovery of uranium and copper from mineralized Dolostone, Gabal Alluga, southwestern Sinai, Egypt. J Radiat Res Appl Sci. https://doi.org/10.1080/16878507.2019.1594095
Feizollahi S, Azizi A (2018) Solvent extraction of copper from an industrial sulfate liquor using Chemorex CP-150. J Min Environ 9(4):905–916. https://doi.org/10.22044/jme.2018.7161.1568
Shabani MA, Irannajad M, Azadmehr AR (2012) Investigation on leaching of malachite by citric acid. Int J Miner Metall Mater 19(9):782. https://doi.org/10.1007/s12613-012-0628-9
El-Sheikh Enass M, Amin Maysa M, Aita Samy K, Rezk Afaf A (2013) Successive recovery of copper and uranium from carbonate-latosol, UM Bogma formation, Abu Thor locality, South Western Sinai, Egypt. Nucl Sci Sci J 2:153–163
Aksu S, Doyle FM (2001) Electrochemistry of copper in aqueous glycine solutions. J Electrochem Soc 148(1):51–57
Eksteen JJ, Oraby EA, Tanda BC (2017) A conceptual process for copper extraction from chalcopyrite in alkaline glycinate solutions. Miner Eng 108:53–66
Remko M, Rode BM (2006) Effect of metal ions (Li+, Na+, K+, Mg2+, Ca2+, Ni2+, Cu2+ and Zn2+) and water coordination on the structure of glycine and zwitterionic glycine. J Phys Chem A 110(5):1960–1967
Kajala A, Gupta OD (2009) Determination of stability constants of As (III) complexes with glycine in Dmf and Dmso at dropping mercury electrode. Rasayan J Chem 2(4):833–835
Oraby EA, Eksteen JJ (2014) The selective leaching of copper from a gold–copper concentrate in glycine solutions. Hydrometallurgy 150:14–19
Eksteen JJ, Oraby EA, Tanda B (2016) An alkaline glycine-based process for copper recovery and iron rejection from chalcopyrite. IMPC 2016—28th International Mineral Processing Congress
Eksteen JJ, Oraby EA, Tanda BC, Tauetsile PJ, Bezuidenhout GA, Newton T, Trask F, Bryan I (2017b) Towards industrial implementation of glycine based leach adsorption technologies for gold-copper ores. In: 7th international world gold conference. 2017, Vancouver, Canada
Tanda BC, Oraby EA, Eksteen JJ (2017) An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 167:153–162
Tanda BC (2017) Glycine as a lixiviant for the leaching of low grade copper-gold ores. Curtin University, Perth
O’Connor GM, Lepkova K, Eksteen JJ, Oraby EA (2018) Electrochemical behaviour of copper in alkaline glycine solutions. Hydrometallurgy 181:221–229
Attia RM, Awny EG (2021) Leaching characterizations and Recovery of Copper and Uranium with glycine solution of sandy dolomite, Allouga area, South Western Sinai, Egypt. Int J Environ Analyt Chem. https://doi.org/10.1080/03067319.2021.2014471
Mokhlis H, Daoudi RD, Azzi M (2021) Selective leaching of copper from waste printed circuit boards (PCBs) using glycine as a complexing agent. Global NEST J 23(1):90–96. https://doi.org/10.30955/gnj.003361
Mądrzak-Litwa I, Borowiak-Resterna A (2019) Solvent extraction of copper(II) from chloride solutions using 1,1′-dialkyl-2,2′-bibenzimidazoles as extractants. Physicochem Probl Min Process 55(5):1165–1178. https://doi.org/10.5277/ppmp19039
Alguacil FJ (1999) Recovery of copper from ammoniacal/ammonium carbonate medium by LIX 973N. Hydrometallurgy 52(1):55–61
Sarangi K, Parhi PK, Padhan E, Palai AK, Nathsarma KC, Park KH (2007) Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using Tbp, LIX 84I and Cyanex 923. Sep Purif Technol 55(1):44–49
Tanda BC, Oraby EA, Eksteen JJ (2017) Recovery of copper from alkaline glycine leach solution using solvent extraction. Sep Purif Technol 178:389–396. https://doi.org/10.1016/j.seppur.2017.06.075
Shapiro L, Brannock WW (1962) Rapid analysis of silicates, carbonates and phosphate rocks. U.S Geol. Surv. Bull., Vol. 114 A
Mathew KJ, Burger S, Ogt SV, Mason PM, Narayanan UI (2009) Uranium assay determination using Davies and Gray titration. Processing the Eighth International Conference on Method and Applications of Radio analytical Chemistry (Marc Viii) Kaailua-Kona, Hawaii,5
Bishr AH (2012) Primary uranium mineralization in paleochannels of the Um Bogma Formation at Allouga, Southwestern Sinai, Egypt. In: 11th Arab Conference on the Peaceful Uses of Atomic Energy. Khartoum. Sudan. Pp 16–20.
Abbas AA (2020) Auriferous, uraniferous and REEs mineralized marl of Middle Um Bogma Formation, Southwestern Sinai, Egypt. J Appl Geol Geophys (IOSR-JAGG) 8(2):12–36. https://doi.org/10.9790/0990-0802031236
Manaa E-S, Negm SH, Abd MO, El-Magied (2018) Alkaline leaching of uranium from El-Sibaiya East Phosphorite in the presence of sodium peroxide. J Nucl Radiochem Sci 18:16–23
Abd El Fattah NA, Al Shami AS, Mohamed SF, El Hady SM (2014) Selective separation of uranium from xenotime-bearing ferruginous sandstone of Southwestern Sinai, by using carbonate leaching. ICAIJ 9(1):19–29
Potgieter GV (2018) Base metal recovery from glycine leach solutions using ion exchange or solvent extraction. B.Sc of Engineering, Faculty of Engineering at Stellenbosch University, p 98
El Hazek MN, El Hefny EA, Boric RM, Ahmed FY, El Kasaby MA, Reda MA (2016) Recovery of alumina and some heavy metals from sulfate liquor. Arab J Chem 9:357–364
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the author(s).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Attia, R.M. Extraction of uranium and copper from acid leached solid waste of Alluoga sedimentary rocks, Southwestern Sinai, Egypt. J Radioanal Nucl Chem 331, 4297–4308 (2022). https://doi.org/10.1007/s10967-022-08467-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08467-y