Abstract
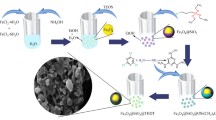
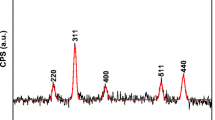
The silica layer protected magnetite (Fe3O4) NPs were functionalized with phosphate group bearing monomer ethylene glycol methacrylate phosphate (EGMP) and its polymer (poly(EGMP) having affinity toward f-element ions. The polymerizable double bonds of EGMP anchored on silica coated magnetite NPs were used to grow poly(EGMP) chains by γ-rays induced polymerization. It was observed in the extraction studies that Pu4+ ions sorbed preferentially in Fe3O4@SiO2-EGMP NPs over UO22+ ions from 3 mol L−1 HNO3. Contrary to this, Fe3O4@SiO2-poly(EGMP) NPs did not exhibit significant selectivity toward UO22+ and Pu4+ ions under similar chemical conditions. Indicating a multiple ligating groups coordination requirement for the binding of UO22+ ions with phosphate groups.









Similar content being viewed by others
References
Munnik P, de Jongh PE, de Jong KP (2015) Recent developments in the synthesis of supported catalysts. Chem Rev 115:6687–6718
Tang S, Zhang H, Lee HK (2015) Advances in sample extraction. Anal Chem 88:228–249
Bai F, Ye G, Chen G et al (2013) New macrocyclic ligand incorporated organosilicas: co-condensation synthesis, characterization and separation of strontium in simulated high level liquid waste. React Funct Polym 73:228–236
Alexandratos SD (2007) New polymer-supported ion-complexing agents: design, preparation and metal ion affinities of immobilized ligands. J Hazard Mater 139:467–470
Grate JW, Egorov OB, O’Hara MJ, DeVol TA (2008) Radionuclide sensors for environmental monitoring: from flow injection solid-phase absorptiometry to equilibration-based preconcentrating minicolumn sensors with radiometric detection. Chem Rev 108:543–562
Alexandratos SD, Crick DW (1996) Polymer-supported reagents: application to separation science. Ind Eng Chem Res 35:635–644
Nguyen T-D, Dinh C-T, Do T-O (2015) Tailoring the assembly, interfaces, and porosity of nanostructures toward enhanced catalytic activity. Chem Commun 51:624–635. https://doi.org/10.1039/C4CC05741D
Xu L, Qi X, Li X et al (2016) Recent advances in applications of nanomaterials for sample preparation. Talanta 146:714–726
Wu B, Zheng N (2013) Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 8:168–197
Wackerlig J, Schirhagl R (2015) Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: a review. Anal Chem 88:250–261
Shwetharani R, Poojashree A, Balakrishna GR, Jyothi MS (2018) La activated high surface area titania float for the adsorption of Pb (ii) from aqueous media. New J Chem 42:1067–1077
La DD, Thi HPN, Nguyen TA, Bhosale SV (2017) Effective removal of Pb(II) using a graphene@ternary oxides composite as an adsorbent in aqueous media. New J Chem 41:14627–14634
Smith SC, Rodrigues DF (2015) Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon N Y 91:122–143
Eigler S, Hirsch A (2014) Chemistry with graphene and graphene oxide—challenges for synthetic chemists. Angew Chemie Int Ed 53:7720–7738
Mauter MS, Elimelech M (2008) Environmental applications of carbon-based nanomaterials. Environ Sci Technol 42:5843–5859
Zhao G, Li J, Ren X et al (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Romanchuk AY, Slesarev AS, Kalmykov SN et al (2013) Graphene oxide for effective radionuclide removal. Phys Chem Chem Phys 15:2321–2327
Chappa S, Singha Deb AK, Ali SM et al (2016) Change in the affinity of ethylene glycol methacrylate phosphate monomer and its polymer anchored on a graphene oxide platform toward uranium(VI) and plutonium(IV) ions. J Phys Chem B 120:2942–2950
Saçmacı Ş, Saçmacı M, Kök C (2018) Grafting of glutathione to magnetic graphene oxide and application for the determination of As (III)/(V) in food samples via a zeta potential analyzer. New J, Chem
Wang X, Liu B, Lu Q, Qu Q (2014) Graphene-based materials: fabrication and application for adsorption in analytical chemistry. J Chromatogr A 1362:1–15
Alimohammady M, Jahangiri M, Kiani F, Tahermansouri H (2017) Highly efficient simultaneous adsorption of Cd (ii), Hg (ii) and As (iii) ions from aqueous solutions by modification of graphene oxide with 3-aminopyrazole: central composite design optimization. New J Chem 41:8905–8919
Buck MR, Schaak RE (2013) Emerging strategies for the total synthesis of inorganic nanostructures. Angew Chemie Int Ed 52:6154–6178
Li N-N, Kang T-F, Zhang J-J et al (2015) Fe3O4@ZrO 2 magnetic nanoparticles as a new electrode material for sensitive determination of organophosphorus agents. Anal Methods 7:5053–5059
Tang SCN, Lo IMC (2013) Magnetic nanoparticles: essential factors for sustainable environmental applications. Water Res 47:2613–2632
Dai Y, Jin J, Zhou L et al (2017) Preparation of hollow SiO2 microspheres functionalized with amidoxime groups for highly efficient adsorption of U (VI) from aqueous solution. J Radioanal Nucl Chem 311:2029–2037
Veliscek-Carolan J, Jolliffe KA, Hanley TL (2013) Selective sorption of actinides by titania nanoparticles covalently functionalized with simple organic ligands. ACS Appl Mater Interfaces 5:11984–11994
Das R, Giri S, Muliwa AM, Maity A (2017) High-performance Hg(II) removal using thiol-functionalized polypyrrole (PPy/MAA) composite and effective catalytic activity of Hg(II)-adsorbed waste material. ACS Sustain Chem Eng 5:7524–7536
Mu X, Qiao J, Qi L et al (2014) Poly (2-vinyl-4, 4-dimethylazlactone)-functionalized magnetic nanoparticles as carriers for enzyme immobilization and its application. ACS Appl Mater Interfaces 6:21346–21354
Soozanipour A, Taheri-Kafrani A, Isfahani AL (2015) Covalent attachment of xylanase on functionalized magnetic nanoparticles and determination of its activity and stability. Chem Eng J 270:235–243
Sharma RK, Dutta S, Sharma S et al (2016) Fe3O4 (iron oxide)-supported nanocatalysts: synthesis, characterization and applications in coupling reactions. Green Chem 18:3184–3209
Baig RBN, Varma RS (2013) Magnetically retrievable catalysts for organic synthesis. Chem Commun 49:752–770
Baeza A, Guillena G, Ramón DJ (2016) Magnetite and metal-impregnated magnetite catalysts in organic synthesis: a very old concept with new promising perspectives. ChemCatChem 8:49–67
Gawande MB, Branco PS, Varma RS (2013) Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem Soc Rev 42:3371–3393
Sharma R, Agrawal VV, Srivastava AK et al (2013) Phase control of nanostructured iron oxide for application to biosensor. J Mater Chem B 1:464–474
Nisticò R, Celi LR, Prevot AB et al (2018) Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J Hazard Mater 342:260–269
Rathod PB, Pandey AK, Meena SS, Athawale AA (2016) Quaternary ammonium bearing hyper-crosslinked polymer encapsulation on Fe3O4 nanoparticles. RSC Adv 6:21317–21325
Böhmer V, Dozol J-F, Grüttner C et al (2004) Separation of lanthanides and actinides using magnetic silica particles bearing covalently attached tetra-CMPO-calix [4] arenes. Org Biomol Chem 2:2327–2334
Rutledge RD, Warner CL, Pittman JW et al (2010) Thiol−Ene induced diphosphonic acid functionalization of superparamagnetic iron oxide nanoparticles. Langmuir 26:12285–12292
Zhang Q, Luan L, Feng S et al (2012) Using a bifunctional polymer for the functionalization of Fe3O4 nanoparticles. React Funct Polym 72:198–205
Nuñez L, Buchholz BA, Vandegrift GF (1995) Waste remediation using in situ magnetically assisted chemical separation. Sep Sci Technol 30:1455–1471
Ojha S, Chappa S, Mhatre AM, et al (2017) Actinides selective extractants coated magnetite nanoparticles for analytical applications. J Radioanal Nucl Chem 312:675–683
Chavan V, Thekkethil V, Pandey AK et al (2014) Assembled diglycolamide for f-element ions sequestration at high acidity. React Funct Polym 74:52–57
O’hara MJ, Carter JC, MacLellan JA et al (2011) Investigation of magnetic nanoparticles for the rapid extraction and assay of alpha-emitting radionuclides from urine: demonstration of a novel radiobioassay method. Health Phys 101:196–208
Urbanova V, Magro M, Gedanken A et al (2014) Nanocrystalline iron oxides, composites, and related materials as a platform for electrochemical, magnetic, and chemical biosensors. Chem Mater 26:6653–6673
Kurzhals S, Zirbs R, Reimhult E (2015) Synthesis and magneto-thermal actuation of iron oxide core—PNIPAM shell nanoparticles. ACS Appl Mater Interfaces 7:19342–19352
Zhang H, McDowell RG, Martin LR, Qiang Y (2016) Selective extraction of heavy and light lanthanides from aqueous solution by advanced magnetic nanosorbents. ACS Appl Mater Interfaces 8:9523–9531
Mutin PH, Guerrero G, Vioux A (2005) Hybrid materials from organophosphorus coupling molecules. J Mater Chem 15:3761–3768
Chappa S, Das S, Debnath AK, et al (2016) Spacer monomer in polymer chain influencing affinity of ethylene glycol methacrylate phosphate toward UO2 2+ and Pu4+ Ions. Ind Eng Chem Res 55:8992–9002
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144
Arsalani N (2010) Synthesis and characterization of PVP-functionalized superparamagnetic Fe3O4 nanoparticles as an MRI contrast agent. eXPRESS Polym Lett 4:329–338. https://doi.org/10.3144/expresspolymlett.2010.42
Wang H, Shao X-Z, Tian Q, Ji Y-Q (2014) Synthesis of TBP-coated magnetic Pst-DVB particles for uranium separation. Nucl Sci Tech 25:22–26
Acknowledgements
Shashikala Ojha is thankful to Dr Pradeepkumar K. S., Associate Director, HS&EG and Head RSSD, BARC and R.K. Gopalakrishnan, Head RHCS, RSSD, BARC for giving permission to carry out doctoral work and their keen interest in the present work. Authors are also thankful to Dr P. K. Pujari, Head Radiochemistry Division, BARC for his keen interest in the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ojha, S., Chappa, S., Mhatre, A.M. et al. Poly(ethylene glycol methacrylate phosphate) grafting on silica shell formed on magnetite nanoparticles: applications to selective sequestration of f-element ions. J Radioanal Nucl Chem 318, 1171–1179 (2018). https://doi.org/10.1007/s10967-018-6228-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6228-z