Abstract
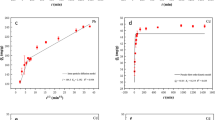
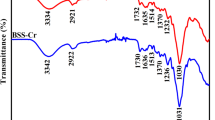
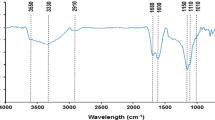
The adsorption of uranium(VI) onto bamboo (Acidosasa longiligula) shoot shell biochar (ASBC) was investigated in batch experiments and the adsorbent was characterized by two-dimensional infrared correlation spectroscopy. The adsorption equilibrium data followed the Langmuir isotherm with maximum adsorption capacity of 32.3 mg g−1 at 298 K. The biochar exhibited a high selectivity for uranium(VI) against Na+ ions. Desorption rate of higher than 90% was obtained over three adsorption–desorption cycles. The adsorption mechanisms of uranium(VI) by ASBC involved surface complexation and cation–π interaction. Results suggested that ASBC was a low-cost and potential adsorbent for uranium(VI) isolation from aqueous solution.















Similar content being viewed by others
References
Sun C, Zhu X, Meng X (2016) Post-Fukushima public acceptance on resuming the nuclear power program in China. Renew Sust Energy Rev 62:685–694
Zhou S, Zhang X (2010) Nuclear energy development in China: a study of opportunities and challenges. Energy 35(11):4282–4288
Lindner H, Schneider E (2015) Review of cost estimates for uranium recovery from seawater. Energy Econ 49:9–22
Bai J, Yin X, Zhu Y, Fan F, Wu X, Tian W, Tan C, Zhang X, Wang Y, Cao S, Fan F, Qin Z, Guo J (2016) Selective uranium sorption from salt lake brines by amidoximated Saccharomyces cerevisiae. Chem Eng J 283:889–895
Zheng M, Zhang Y, Liu X, Qi W, Kong F (2016) Progress and prospects of salt lake research in China. Acta Geol Sin Engl 90(4):1195–1235
Fan F, Bai J, Fan F, Yin X, Wang Y, Tian W, Wu X, Qin Z (2014) Solvent extraction of uranium from aqueous solutions by α-benzoinoxime. J Radioanal Nucl Chem 300(3):1039–1043
Chen Y, Wei Y, He L, Tang F (2016) Separation of thorium and uranium in nitric acid solution using silica based anion exchange resin. J Chromatogr A 1466:37–41
Burns AD, Abbasi P, Dreisinger DB (2016) Uranous sulfate precipitation as a novel hydrometallurgical process for uranium purification. Hydrometallurgy 163:49–54
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Bhalara PD, Punetha D, Balasubramanian K (2014) A review of potential remediation techniques for uranium(VI) ion retrieval from contaminated aqueous environment. J Environ Chem Eng 2(3):1621–1634
Wang G, Liu J, Wang X, Xie Z, Deng N (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168(2–3):1053–1058
Lehmann J (2007) A handful of carbon. Nature 447(7141):143–144
Novak J, Ro K, Ok YS, Sigua G, Spokas K, Uchimiya S, Bolan N (2016) Biochars multifunctional role as a novel technology in the agricultural, environmental, and industrial sectors. Chemosphere 142:1–3
Hu H, Zhang J, Lu K, Tian Y (2015) Characterization of Acidosasa edulis shoot shell and its biosorption of copper ions from aqueous solution. J Environ Chem Eng 3(1):357–364
Kumar S, Loganathan VA, Gupta RB, Barnett MO (2011) An Assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J Environ Manag 92(10):2504–2512
Zhang Z, Cao X, Liang P, Liu Y (2012) Adsorption of uranium from aqueous solution using biochar produced by hydrothermal carbonization. J Radioanal Nucl Chem 295(2):1201–1208
Yakout SM (2015) Effect of porosity and surface chemistry on the adsorption–desorption of uranium(VI) from aqueous solution and groundwater. J Radioanal Nucl Chem 308(2):555–565
Ashry A, Bailey EH, Chenery SR, Young SD (2016) Kinetic study of time-dependent fixation of UVI on biochar. J Hazard Mater 320:55–66
Hadjittofi L, Pashalidis I (2014) Uranium sorption from aqueous solutions by activated biochar fibres investigated by FTIR spectroscopy and batch experiments. J Radioanal Nucl Chem 304(2):897–904
Noda I (2014) Frontiers of two-dimensional correlation spectroscopy. Part 2. Perturbation methods, fields of applications, and types of analytical probes. J Mol Struct 1069:23–49
Noda I (1990) Two-dimensional infrared (2D IR) spectroscopy: theory and applications. Appl Spectrosc 44(4):550–561
Al Lafi AG, Al Abdullah J (2015) Cesium and cobalt adsorption on synthetic nano manganese oxide: a two dimensional infra-red correlation spectroscopic investigation. J Mol Struct 1093:13–23
Zhang J, Chen L, Yin H, Jin S, Liu F, Chen H (2017) Mechanism study of humic acid functional groups for Cr(VI) retention: two-dimensional FTIR and 13C CP/MAS NMR correlation spectroscopic analysis. Environ Pollut 225:86–92
Hu H, Jiang B, Zhang J, Chen X (2015) Adsorption of perrhenate ion by bio-char produced from Acidosasa edulis shoot shell in aqueous solution. RSC Adv 5(127):104769–104778
Mohan D, Rajput S, Singh VK, Steele PH, Pittman CU Jr (2011) Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J Hazard Mater 188(1–3):319–333
Azargohar R, Nanda S, Kozinski JA, Dalai AK, Sutarto R (2014) Effects of temperature on the physicochemical characteristics of fast pyrolysis bio-chars derived from Canadian waste biomass. Fuel 125:90–100
Fang Q, Chen B, Lin Y, Guan Y (2014) Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ Sci Technol 48(1):279–288
Wang Z, Guo H, Shen F, Yang G, Zhang Y, Zeng Y, Wang L, Xiao H, Deng S (2015) Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4 +), nitrate (NO3 −), and phosphate (PO4 3−). Chemosphere 119:646–653
Wu W, Yang M, Feng Q, McGrouther K, Wang H, Lu H, Chen Y (2012) Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 47:268–276
Belgacem A, Rebiai R, Hadoun H, Khemaissia S, Belmedani M (2014) The removal of uranium (VI) from aqueous solutions onto activated carbon developed from grinded used tire. Environ Sci Pollut Res 21(1):684–694
Manos MJ, Kanatzidis MG (2012) Layered metal sulfides capture uranium from seawater. J Am Chem Soc 134(39):16441–16446
Noda I, Dowrey AE, Marcott C, Story GM, Ozaki Y (2000) Generalized two-dimensional correlation spectroscopy. Appl Spectrosc 54(7):236A–248A
Tytłak A, Oleszczuk P, Dobrowolski R (2015) Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ Sci Pollut Res 22(8):5985–5994
Hu H, Jiang B, Wu H, Zhang J, Chen X (2016) Bamboo (Acidosasa edulis) shoot shell biochar: its potential isolation and mechanism to perrhenate as a chemical surrogate for pertechnetate. J Environ Radioact 165:39–46
Shen Z, Jin F, Wang F, McMillan O, Al-Tabbaa A (2015) Sorption of lead by Salisbury biochar produced from British broadleaf hardwood. Bioresour Technol 193:553–556
Dong X, Ma LQ, Li Y (2011) Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J Hazard Mater 190(1–3):909–915
Chen T, Zhou Z, Han R, Meng R, Wang H, Lu W (2015) Adsorption of cadmium by biochar derived from municipal sewage sludge: impact factors and adsorption mechanism. Chemosphere 134:286–293
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res 46(3):854–862
Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res Int 20(1):358–368
Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou Y, Zheng B, Cai X (2016) Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: performance and mechanism. RSC Adv 6(7):5223–5232
Wang Z, Liu G, Zheng H, Li F, Ngo HH, Guo W, Liu C, Chen L, Xing B (2015) Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour Technol 177:308–317
Deveci H, Kar Y (2013) Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. J Ind Eng Chem 19(1):190–196
Gondhalekar SC, Shukla SR (2014) Equilibrium and kinetics study of uranium(VI) from aqueous solution by Citrus limetta peels. J Radioanal Nucl Chem 302(1):451–457
Yang J, Volesky B (1999) Biosorption of uranium on Sargassum biomass. Water Res 33(15):3357–3363
Ho YS, Mckay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34(3):735–742
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Li Y, Yang L, Liu X, Li N, Zhang L, Li Q, Yang Y, Duan Y, Zhang F (2015) Highly enhanced selectivity for the separation of rhenium and molybdenum using amino-functionalized magnetic Cu-ferrites. J Mater Sci 50(18):5960–5969
Febrianto J, Kosasih AN, Sunarso J, Ju Y-H, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162(2–3):616–645
Kiran I, Akar T, Ozcan AS, Ozcan A, Tunali S (2006) Biosorption kinetics and isotherm studies of Acid Red 57 by dried Cephalosporium aphidicola cells from aqueous solutions. Biochem Eng J 31(3):197–203
Farooq U, Kozinski JA, Khan MA, Athar M (2010) Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour Technol 101(14):5043–5053
Yi Z, Yao J, Kuang Y, Chen H, Wang F, Xu J (2014) Uptake of hexavalent uranium from aqueous solutions using coconut husk activated carbon. Desalin Water Treat 57(4):1749–1755
Zhu M, Liu R, Chai H, Yao J, Chen Y, Yi Z (2016) Hazelnut shell activated carbon: a potential adsorbent material for the decontamination of uranium(VI) from aqueous solutions. J Radioanal Nucl Chem 310(3):1147–1154
Kütahyalı C, Eral M (2010) Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. J Nucl Mater 396(2–3):251–256
Caccin M, Giacobbo F, Da Ros M, Besozzi L, Mariani M (2012) Adsorption of uranium, cesium and strontium onto coconut shell activated carbon. J Radioanal Nucl Chem 297(1):9–18
Morsy AMA, Hussein AEM (2011) Adsorption of uranium from crude phosphoric acid using activated carbon. J Radioanal Nucl Chem 288(2):341–346
Dong L, Yang J, Mou Y, Sheng G, Wang L, Linghu W, Asiri AM, Alamry KA (2017) Effect of various environmental factors on the adsorption of U(VI) onto biochar derived from rice straw. J Radioanal Nucl Chem 314(1):377–386
Tian G, Geng J, Jin Y, Wang C, Li S, Chen Z, Wang H, Zhao Y, Li S (2011) Sorption of uranium(VI) using oxime-grafted ordered mesoporous carbon CMK-5. J Hazard Mater 190(1–3):442–450
Duranoğlu D, Trochimczuk AW, Beker U (2012) Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile–divinylbenzene copolymer. Chem Eng J 187:193–202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, H., Zhang, X., Wang, T. et al. Bamboo (Acidosasa longiligula) shoot shell biochar: its potential application to isolation of uranium(VI) from aqueous solution. J Radioanal Nucl Chem 316, 349–362 (2018). https://doi.org/10.1007/s10967-018-5731-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5731-6