Abstract
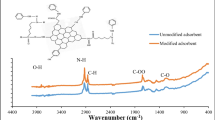
Aqueous strontium biosorption using aerobic granules was investigated. Parameters affecting the biosorption were optimized, including initial pH, biomass dosage, temperature, and rotation speed. The equilibrium data were fitted using Langmuir and Freundlich models, and both could well describe the process (R 2 = 0.987 and 0.989, respectively). Ion exchange and water-desorption experiments were conducted, and ion exchange together with physical adsorption were found to be the main mechanisms. The aerobic granules were characterized with methods including scanning electron microscopy, X-ray photoelectron spectroscopy, and Fourier transform infrared spectroscopy. The results showed that surface complexation could also be involved in the Sr(II) biosorption.











Similar content being viewed by others
References
Chegrouche S, Mellah A, Barkat A (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235:306–318
Inan S, Altas Y (2010) Adsorption of strontium from acidic waste solution by Mn-Zr mixed hydrous oxide prepared by co-precipitation. Sep Sci Technol 45:269–276
Casacuberta N, Masque P, Garcia-Orellana J, Garcia-Tenorio R, Buesseler KO (2013) Sr-90 and Sr-89 in seawater off Japan as a consequence of the Fukushima Dai-ichi nuclear accident. Biogeosciences 10:3649–3659
Khani MH, Pahlavanzadeh H, Alizadeh K (2012) Biosorption of strontium from aqueous solution by fungus Aspergillus terreus. Environ Sci Pollut Res 19:2408–2418
Wang L, Wan C, Lee DJ, Tay JH, Chen X, Liu X, Zhang Y (2013) Adsorption–desorption of strontium from waters using aerobic granules. J Taiwan Inst Chem Eng 44:454–457
Galamboš M, Osacký M, Rosskopfová O, Krajňák A, Rajec P (2012) Comparative study of strontium adsorption on dioctahedral and trioctahedral smectites. J Radioanal Nucl Chem 293:889–897
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium (II) and barium (II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Wallace SH, Shaw S, Morris K, Small JS, Fuller AJ, Burke IT (2012) Effect of groundwater pH and ionic strength on strontium sorption in aquifer sediments: implications for Sr-90 mobility at contaminated nuclear sites. Appl Geochem 27:1482–1491
Kumar S, Sivaiah M, Venkatesan K, Krishna R, Murthy G, Sasidhar P (2003) Removal of cesium and strontium from acid solution using a composite of zirconium molybdate and zirconium tungstate. J Radioanal Nucl Chem 258:321–327
Behrens EA, Clearfield A (1997) Titanium silicates, M3HTi4O4(SiO4)(3)center dot 4H(2)O (M = Na+, K+), with three-dimensional tunnel structures for the selective removal of strontium and cesium from wastewater solutions. Micropor Mesoporous Mat 11:65–75
Nakayama S, Itoh K (2003) Immobilization of strontium by crystalline zirconium phosphate. J Eur Ceram Soc 23:1047–1052
Kapoor A, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Bioresource Technol 61:221–227
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Fein JB, Daughney CJ, Yee N, Davis TA (1997) A chemical equilibrium model for metal adsorption onto bacterial surfaces. Geochim Cosmochim Acta 61:3319–3328
Liu Y, Yang SF, Tan SF, Lin YM, Tay JH (2002) Aerobic granules: a novel zinc biosorbent. Lett Appl Microbiol 35:548–551
Yao L, Ye ZF, Tong MP, Lai P, Ni JR (2009) Removal of Cr3+ from aqueous solution by biosorption with aerobic granules. J Hazard Mater 165:250–255
Xu H, Liu Y (2008) Mechanisms of Cd2+, Cu2+ and Ni2+ biosorption by aerobic granules. Sep Purif Technol 58:400–411
Wang XH, Song RH, Teng SX, Gao MM, Ni JY, Liu FF, Wang SG, Gao BY (2010) Characteristics and mechanisms of Cu (II) biosorption by disintegrated aerobic granules. J Hazard Mater 179:431–437
Nancharaiah Y, Joshi H, Mohan T, Venugopalan V, Narasimhan S (2006) Aerobic granular biomass: a novel biomaterial for efficient uranium removal. Curr Sci India 91:503–509
Wang J, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451
Sun F, Sun WL, Sun HM, Ni JR (2011) Biosorption behavior and mechanism of beryllium from aqueous solution by aerobic granule. Chem Eng J 172:783–791
Liu Y, Xu H, Yang SF, Tay JH (2004) A theoretical model for biosorption of cadmium, zinc and copper by aerobic granules based on initial conditions. J Chem Technol Biot 79:982–986
Ozdes D, Gundogdu A, Kemer B, Duran C, Kucuk M, Soylak M (2014) Assessment of kinetics, thermodynamics and equilibrium parameters of Cr(VI) biosorption onto Pinus brutia Ten. Can J Chem Eng 92:139–147
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich H (1906) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Gok C, Gerstmann U, Aytas S (2013) Biosorption of radiostrontium by alginate beads: application of isotherm models and thermodynamic studies. J Radioanal Nucl Chem 295:777–788
Yavari R, Huang Y, Mostofizadeh A (2010) Sorption of strontium ions from aqueous solutions by oxidized multiwall carbon nanotubes. J Radioanal Nucl Chem 285:703–710
İnan S, Altaş Y (2010) Adsorption of strontium from acidic waste solution by Mn–Zr mixed hydrous oxide prepared by co-precipitation. Sep Sci Technol 45:269–276
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235:306–318
Ji YQ, Hu YT, Tian Q, Shao XZ, Li J, Safarikova M, Safarik I (2010) Biosorption of strontium ions by magnetically modified yeast cells. Sep Sci Technol 45:1499–1504
Wang L, Wan C, Lee DJ, Liu X, Zhang Y, Chen XF, Tay JH (2014) Biosorption of antimony(V) onto Fe(III)-treated aerobic granules. Bioresour Technol 158:351–354
Ren TT, Liu L, Sheng GP, Liu XW, Yu HQ, Zhang MC, Zhu JR (2008) Calcium spatial distribution in aerobic granules and its effects on granule structure, strength and bioactivity. Water Res 42:3343–3352
Sun XF, Wang SG, Liu XW, Gong WX, Bao N, Gao BY (2008) Competitive biosorption of zinc (II) and cobalt (II) in single-and binary-metal systems by aerobic granules. J Colloid Interface Sci 324:1–8
Acknowledgement
This study is supported by the State Key Laboratory of Pollution Control and Resource Reuse Foundation (PCRRF14003).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, L., Liu, X., Chen, Xf. et al. Biosorption of Sr(II) from aqueous solutions using aerobic granules: equilibrium and mechanisms. J Radioanal Nucl Chem 306, 193–202 (2015). https://doi.org/10.1007/s10967-015-4084-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4084-7