Abstract
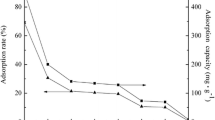
The sorption of uranium onto ZrP2O7 in the presence of oxalic acid has been investigated as a function of temperature (20, 40 and 60 °C). Using several complementary analytical methods to characterize the solid surface, it has been shown that the oxalic acid interact with the zirconium diphosphate affecting its surface reactivity. A significant influence of temperature on the sorption reaction has been revealed in the batch experiments. Temperature dependence sorption data and microcalorimetric measurements have been used to determine enthalpy change associated to the sorption reaction. The results have shown that oxalic acid has an important effect on uranium sorption, which is more evident at 60 °C.






Similar content being viewed by others
References
IAEA (2006) Geological Disposal of Radioactive Waste, Safety Requeriments No. WS-R-4, Vienna, pp 49
Reiller P, Moulin V, Casanova F, Dautel C (2003) On the study of Th(IV)-humic acid interactions by competition sorption studies with silica and determination of global interaction constants. Radiochim Acta 91:513–524
Kumar S, Tomar BS, Ramanathan S, Manchanda VK (2006) Effect of humic acid on cesium sorption on silica colloids. Radiochim Acta 94:369–373
Schott J, Acker M, Barkleit A, Brendler V, Taut S, Bernhard G (2012) The influence of temperature and small organic ligands on the sorption of Eu(III) on Opalinus Clay. Radioch. Acta 100:315–324
Murphy RJ, Lenthart JJ, Honeyman BD (1999) The sorption of thorium (IV) and uranium (VI) to hematite in the presence of natural organic matter. Colloid and Surfaces A:Physicochemical and Engineering Aspects 157:47–62
Hanks TC, Winograd IJ, Anderson RE, Reilly TE, Weeks EP (1999) Yucca Mountain as a Radioactive-Waste Repository. USGS Circular 1184:19
Verstricht J, Blumling P, Merceron T (2003) Repository concepts for nuclear waste disposal in clay formations. In: Myrvoll F (ed) Proceedings of the 6th International Symposium on Field measurements in geomechanic, Oslo, pp 826
Angove MJ, Johnson BB, Wells JD (1998) The influence of temperature on the adsorption of cadmium (II) and cobalt (II) on kaolinite. J. Coll. Interf. Sc. 204:93–103
Echeverria J, Indurain J, Churio E, Garrido J (2003) Simultaneous effect of pH, temperature, ionic strength and initial concentration on the retention of Ni on illite. Coll. Surf. A: Physicochem. Eng. Asp. 218:1–13
Tertre E, Berger G, Castet S, Loubet M, Simoni E (2005) Experimental sorption of Ni2+, Cs+ and Ln3+ onto a montmorillonite up to 150°C. Geochim Cosmochim Acta 69:4937–4948
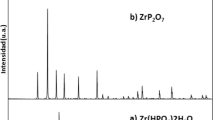
Finck N, Drot R, Mercier-Bion F, Simoni E, Catalette H (2007) Temperature effects on the acidity properties of zirconium diphosphate. J Colloid Interface Sci 321:230–236
Almazan-Torres MG, Drot R, Mercier-Bion F, Catalette H, Auwer CD, Simoni E (2008) Surface complexation modeling of uranium (VI) sorbed onto zirconium oxophosphate versus temperature: thermodynamic and structural approaches. J Colloid Interface Sci 323:42–51
Qian LJ, Hu PZ, Jiang ZJ, Geng YX, Wu WS (2010) Effect of pH, fulvic acid and temperature on the sorption of uranyl on ZrP2O7. Science Chine: Chemistry 53(6):1429–1437
García-González N, Ordoñez-Regil E, Simoni E, Barrera-Díaz CE (2009) Effect of organic acids on sorption of uranyl ions in solution by ZrP2O7. J. Radionalytical and Nuclear Chemistry 283:409–415
García-González N, Ordoñez-Regil E, Almazán-Torres MG, Solis D, Simoni E (2012) Speciation of U(VI) sorbed onto ZrP2O7 in the presence of citric and oxalic acid. Radiochim Acta 100:305–309
Noh JS, Schwarz JA (1989) Estimation of the Point of Zero Charge of Simple Oxides by Mass Titration. J Colloid Interface Sci 130:157–164
Langford JI, Wilson AJC (1978) Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Cryst. 11:102–113
Martin J.D. (2008) Xpowder12: A software package for powder X-ray Diffraction Analysis, www.xpowder.com, pp 153
Karasyova ON, Ivanova LI, Lakshtanov LZ, Lovgren L (1999) Strontium sorption on hematite at elevated temperatures. J. Coll. Interf. Sci. 220:419–728
Morel JP, Marmier N, Hurel Ch, Morel-Desrosiers N (2012) Effect of temperature on the sorption of europium on alumina: microcalorimetry and batch experiments. J Colloid Interface Sci 376:196–201
Ho YS, McKay G (1999) Pseudo-second order model for soprtion process. Process Biochem 34:451–465
Ho YS, McKay G (2000) The kinetics of Sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Ho YS, Ofomaja AE (2006) Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. Journal of Hazardous Materials B 129:137–142
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García González, N., Ordóñez Regil, E., Solís Casados, D.A. et al. The effect of temperature on the sorption of U(VI) onto ZrP2O7 in the presence of oxalic acid. J Radioanal Nucl Chem 301, 483–491 (2014). https://doi.org/10.1007/s10967-014-3158-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3158-2