Abstract
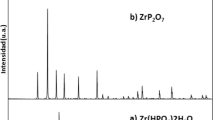
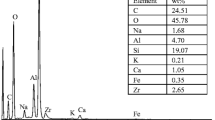
Hydration of zirconium diphosphate (ZrP2O7) conduced to formation of active sites in solid/liquid interface. In ZrP2O7/NaClO4 0.5 M suspensions, active sites and their acidity constants are quite determined but the presence of some impurities is now studied. This work was conducted to determine the surface properties changes produced by oxalic and citric acid during the hydration process. Moreover the presence of organic acids with ZrP2O7 modified reveals an increase in uranium sorption constants. The zirconium diphosphate has been characterized using X-ray powder diffraction (XRD), Scanning electron microscopy (SEM) and Particle induced X-ray emission and Neutron (PIXE). Furthermore, the specific surface area, measured by the BET method, was 3.5 m2/g. The pH corresponding to the isoelectric point, determined by Zeta Potential measurements and mass titration was 3.6. The sites density calculated using titration curves was around of 5.37 s/nm2 for NaClO4 0.5 M, 13.71 s/nm2 for NaClO4 0.5 M/citric acid 0.1 M and 7.33 s/nm2 NaClO4 0.5 M/oxalic acid 0.1 M. The surface acidity constants and species distribution in surface were calculated by means of simulation of the titration curves with the FITEQL code (constant capacitance model), for ZrO and PO amphoteric sites of ZrP2O7. The uranyl sorption edge was determined for NaClO4 0.5 M. It spreads between pH 3 and 4.5 for complete sorption according to the previously published results. In the ZrP2O7–citrate modified surface, the uranyl sorption edge begin at pH 2 and was almost complete at pH 3.2 while ZrP2O7–oxalate modified surface edge started at 50% of sorption at pH of 1.5 and was complete at pH 3.




Similar content being viewed by others
References
Guillaumont R (1994) Radiochemical approaches to the migration of elements from a radwaste repository. Radiochim Acta 66(67):231–242
De Marsily G (1988) Radionuclide migration in the geosphere: an overview Radiochim Acta 44/45:159
Powell RM Puls RW, Blowes DW, Vogan JL, Gillham RW, Schultz D, Powell PP, Sivavic T, Landis R (1998) Permable reactive barrier technologies for contaminant remediation. Technical report, EPA/600/R-98/125. Office of Research and Development, Office of Solid Waste and Emergency Response, U.S. EPA, Washington, DC
Simon FG, Meggyes T (2000) Removal of organic and inorganic pollutants from groundwater using permeable reactive barriers. Pub Land Contam Reclam 8:103–116
Drot R, Simoni E (1999) Uranium(VI) and europium(III) speciation at the phosphate compounds-solution interface. Langmuir 15:4820–4827
Drot R, Simoni E, Alnot M, Ehrhardt JJ (1998) Structural environment of uranium (VI) and europium (III) species sorbed onto phosphate surfaces: XPS and optical spectroscopy studies. J Colloid Interface Sci 205:410–416
Hering JG, Kraemer S (1994) Kinetics of complexation reactions at surfaces and in solution: implications for enhanced radionuclide migration. Radiochim Acta 66/67:63
Finck N, Drot R, Lagarde G, Mercier-Bion F, Catalette H, Simoni E (2008) Temperature effects on the interaction mechanisms between U(VI) and Eu(III) and ZrP2O7: experiment and modeling. Radiochim Acta 96:11–21
Finck N, Drot R, Mercier-Bion F, Simoni E, Catalette H (2007) Temperature effects on the surface acidity properties of zirconium diphosphate. J Colloid Interface Sci 312:230–236
Moulin V (2005) Complexation of radionuclides with humic substances. In: Use of humic substances to remediate polluted environments: from theory to practice, Vol 7, pp 155–173
Institut für Nucleare Entsorgung (2000) Influence of humic acids on the migration behaviour of radioactive and non-radioactive substances under conditions close to nature: Metal-Ion behaviour in water/mineral system. Final Report
Perminova IV, Hatfield K (2005) Remediation chemistry of humic substances: theory and implications for technology. In: Use of humic substances to remediate polluted environments: From Theory to Practice, Vol 1, pp 3–36
Makhfouk ME, Meray ME, Castetbon A, Astruc M (2002) Effect of citrate, oxalate and pyrophosphate ligands on the electrochemical reduction of the uranyl ion in perchloric acid medium. Bull Electrochem 18(2):63–70
Tadanier CJ, Matthew JE (2002) Formulating the charge-distribution multisite surface complexation model using FITEQL. Soil Sci Soc Am J 66:1505–1517
Davis JA, Kent DB (1990) Surface complexation modeling in aqueous geochemistry. In Hochella MF Jr, White AF (eds) Review in mineralogy: mineral water interface geochemistry, Vol 23. Mineralogical Society Americana, Washington, DC, pp 177–260
Drot R, Lindecker C, Fourest B, Simoni E (1998) Surface characterization of zirconium and thorium phosphate compounds. New J Chem 22:1105–1109
Dacheux N, Brandel V, Genet M (2001) Studies on the chemistry of uranium and thorium phosphates. Thorium phosphate diphosphate: a matrix for storage of radioactive wastes. Radiochemistry 43(1):16–24
Masse R, Grenier J-C (1971) Bull Soc Fr Min Cristallogr 94:437
Bjorklund CW (1958) Solid-state synthesis of monazite-type compounds. J Am Chem Soc 79:6347
Markert B (1996) Instrumental element and multi-element analysis of plant samples, methods and applications. Willey & Sons, New York
Woldseth R (1973) X-ray energy spectrometry. Kevex Corporation, Burlingame, California
Friedlander G (1981) Nuclear and radiochemistry. Wiley, New York, pp 208–209
Whitehouse MJ (2003) Geochronology: linking the isotopic record with petrology and textures. Geological Society of London, pp 50
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Noh JS, Schwarz JA (1989) Estimation of the point of zero charge of simple oxides by mass titration. J Colloid Interface Sci 130:157–164
Nagy NM, Kónya J (2007) Study of pH-dependant charges of soils by surface acid-base properties. J Colloid Interface Sci 305:94–100
Herbelin AL, Westall JC (1996) FITEQL: a program for the determination of chemical equilibrium constants from experimental data. Report 96-01. Department of Chemistry, Oregon State University, Corvallis
Lomenech C, Drot R, Simoni E (2003) Speciation of uranium (VI) at the solid/solution interface: sorption modeling on zirconium silicate and zirconium oxide. Radiochim Acta 91:453–461
Balche GU, Georgi A, Woszidlo S, Kopinke FD, Poerchmann J (2005) Utilization of immobilized humic organic matter for in situ subsurface remediation. In: Perminova IV et al (eds) Use of humic substances to remediate polluted environments. Springer, Netherlands, pp 203–232
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia-Gonzalez, N., Ordóñez-Regil, E., Simoni, E. et al. Effect of organic acids on sorption of uranyl ions in solution onto ZrP2O7 . J Radioanal Nucl Chem 283, 409–415 (2010). https://doi.org/10.1007/s10967-009-0406-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0406-y