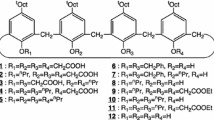
Abstract
Calixarenes has been subject to extensive research in development of many extractants, transporters, stationary phases, electrode ionophores and optical and electrochemical sensors over the past four decades. In this paper, the nuclear applications of calixarenes are summarized in six fields including complexation studies, solvent extraction, membrane transport, chromatography, luminescent and colorimetric applications, and electroanalytical applications. In the first to fourth sections, the extractability, extraction equilibria and extraction constants of lanthanide, actinide and other nuclear waste cations ions, which were subjected to solvent extraction by the macrocyclic ligands, are reviewed. In two last sections, the analytical applications of calixarene complexes towards nuclear waste cations, including spectroscopic and electroanalytic sensors, are discussed. The examples described in this review illustrate the potential of calixarene derivatives in the rapidly growing field of cations recognition in nuclear wastes.




Similar content being viewed by others
References
Sliwa W (2002) Calixarene complexes with transition metal, lanthanide and actinide ions. Croatica Chemica Acta 75(1):131–153
Saeid Hosseini M, Hosseini-Bandegharaei A (2010) Selective extraction of Th(IV) over U(VI) and other co-existing ions using eosin B-impregnated Amberlite IRA-410 resin beads. J Radioanal Nucl Chem 283:23–30
Makrlik E, Vanura P, Selucky P (2010) Solvent extraction of microamounts of europium and americium into nitrobenzene by using synergistic mixture of hydrogen dicarbollylcobaltate and 1,2-(diphenylphosphino)ethylene dioxide. J Radioanal Nucl Chem 283:45–50
Ren X, Wang S, Yang S, Li J (2010) Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX-80 bentonite. J Radioanal Nucl Chem 283:253–259
Zuo C, Yan T, Zheng W, Li C, Wang X (2010) Kinetics and mechanism of stripping of Np(IV) by acetohydroxamic acid using a Lewis cell. J Radioanal Nucl Chem 283:83–87
Jung J, Cho YH, Hahn PS (1999) Scavenging of UO2 2+ using 4-sulfonic calix[6]arene in the presence of goethite. J Radioanal Nucl Chem 242(3):635–639
Malkhede DD, Dhadke PM, Khopkar SM (1999) Liquid-liquid extraction of thorium (IV) with hexaacetato calix[6]arene. J Radioanal Nucl Chem 241(1):179–182
Schmeide K, Heise KH, Bernhard G, Keil D, Jansen K (2004) Uranium(VI) separation from aqueous solution by calix[6]arene modified textiles. J Radioanal Nucl Chem 261(1):61–67
Montavon G, Duplâtre G, Asfari Z, Vicens J (1996) Effect of alkali ions (Na+, K+) on the solvent extraction of uranium(VI) with a di-carboxylated calix[4]arene. J Radioanal Nucl Chem 210(1):87–103
Grunder M, Dozol JF, Asfari Z, Vicens J (1999) Simultaneous removal of technetium and cesium by functionalized calixarenes from acidic liquid waste. J Radioanal Nucl Chem 241(1):59–67
Rogers RD, Bauer CB (1996) Water soluble calixarenes as possible metal ion extractants in polyethylene glycol-based aqueous biphasic systems. J Radioanal Nucl Chem 208(1):153–161
Baglan N, Dinse C, Cossonnet C, Abidi R, Asfari Z (1997) Investigation of U(VI) extraction with calixarene: application to analysis of urine sample. J Radioanal Nucl Chem 226(1–2):261–265
Makrlik E, Budka J, Vanura P, Selucky P (2008) Extraction of Ba2+, Pb2+ and Cd2+ into nitrobenzene by using strontium dicarbollylcobaltate in the presence of tetramethyl p-tert-butylcalix[4]arene tetraketone. J Radioanal Nucl Chem 277(2):487–490
Makrlik E, Vanura P (2005) Solvent extraction of barium picrate by p-tert-butylcalix[4]arene-tetrakis (N,N-diethylacetamide). J Radioanal Nucl Chem 267(3):699–701
Du HF, Zhang AY, Yang ZX, Zhou ZM (1999) Solvent extraction of uranium(VI) by p-tert-butylcalix[n]-arene acetate. J Radioanal Nucl Chem 241(1):241–243
Kyrs M, Svoboda K, Lhotak P, Alexova J (2003) Synergistic solvent extraction of Eu, Sr and Cs into chlorobenzene solutions of the three conformers of tetrathiocalixarene and dicarbollide. J Radioanal Nucl Chem 258(3):497–509
Makrlik E, Vanura P (2008) Stability constants of complexes of Li+, H3O+, NH4 + and Ag+ with hexaethyl calix[6]arene hexaacetate in nitrobenzene saturated with water. J Radioanal Nucl Chem 277(2):495–497
Makrlik E, Vanura P (2006) Stability of the p-tert-butylcalix[4]arene-tetrakis (N, N-diethylacetamide)-hydrogen cation complex in water saturated nitrobenzene. J Radioanal Nucl Chem 268(1):163–165
Makrlik E, Vanura P (2005) Stability of the ammonium–p-tert -butylcalix[4]arene-tetrakis (N,N-diethylacetamide) complex in nitrobenzene saturated with water. J Radioanal Nucl Chem 267(3):711–713
Gharib F, Taghvaei-Ganjali S, Eslamipanah M, Mazooji R, Ebrahimi S (2006) Complexation of di and tetra-benzyloxy ether derivatives of calix[4]arene with alkali metal cations. Acta Chim Slov 53:424–427
Yaftian MR, Abdollahi H, Shokouhi R, Tavakoli M, Matt D (2007) Ion binding properties of 5,11,17,23-tetra-tert-Butyl-25,27-bis(diethylcarbamoylmethoxy)-26,28-bis(diphenylphosphinoylmethoxy)calix[4]arene towards alkaline-earth cations. Chemia Analityczna 52(1):103–113
Sliwa W, Girek T (2010) Calixarene complexes with metal ions. J Incl Phenom Macrocycl Chem 66(1–2):15–41
Salorinne K, Nissinen M (2008) Calixcrowns: synthesis and properties. J Incl Phenom Macrocycl Chem 61(1–2):11–27
Kim JS, Vicens J (2009) Progress of calixcrowns chemistry. J Incl Phenom Macrocycl Chem 63(1–2):189–193
Ashram M (2006) Syntheses of novel hexahomotrithiacalix[3]arenes. J Incl Phenom Macrocycl Chem 54(3–4):253–259
Deligöz H (2006) Azocalixarenes: synthesis, characterization, complexation, extraction, absorption properties and thermal behaviours. J Incl Phenom Macrocycl Chem 55(3–4):197–218
Mellah B, Abidi R, No K (2010) Complexation of metal ions by ethylester derivative of p-tetraphenyl tetrahomodioxacalix[4]arene. J Incl Phenom Macrocycl Chem 66(1–2):49–54
Mellah B, Abidi R, Herchbach H, No K, Kim JS, Arnaud F, Veronique H (2010) Interaction between p-tetraphenyl tetrahomodioxacalix[4]arene amide derivatives and alkali and alkaline-earth metal cations. J Incl Phenom Macrocycl Chem 66(1–2):153–161
Mariani M, Macerata E, Galletta M, Buttafava A, Casnati A, Ungaro R, Faucitano A, Giola M (2007) Partitioning of minor actinides: effects of gamma irradiation on the extracting capabilities of a selected calixarene-based picolinamide ligand. Radiat Phys chem 76:1285–1289
Leydier A, Lecercle D, Pellet-Rostaing SP, Guillon AFR, Taran F, Lemaire M (2008) Sequestering agents for uranyl chelation: new calixarene ligands. Tetrahedron 64:11319–11324
Mewis RE, Archibald SJ (2010) Biomedical applications of macrocyclic ligand complexes, (Review). Coord Chem Rev 254(15–16):1686–1712
Karavan M, Arnaud-Neu F, Hubscher-Bruder V, Smirnov I, Kalchenko V (2010) Novel phosphorylated calixarenes for the recognition of f-elements. J Incl Phenom Macrocycl Chem 66(1–2):113–123
Tanaka T, Iki N, Kajiwara T, Yamashita M, Hoshino H (2009) One-step heterogeneous assembly of terbium(III) and silver(I) with thiacalix[4]arene ligands to form a cage including terbium(III) in an octa-oxygen cube. J Incl Phenom Macrocycl Chem 64(3–4):379–383
Tomisic V, Galic N, Bertosa B, Frkanec L, Simeon V, Zinic M (2005) hydrogen bonding and solvent effects on complexation of alkali metal cations by lower rim calix[4]arene tetra(O-[N-acetyl-D-phenylglycine methyl ester]) derivative. J Incl Phenom Macrocycl Chem 53(3):263–268
Custelcean R, Delmau LH, Moyer BA, Sessler JL, Cho WS, Gross D, Bates GW, Brooks SJ, Light ME, Gale PA (2005) Calix[4]pyrrole: an old yet new ion-pair receptor. Angew Chem Int Ed 44(17):2537–2542
Arora V, Chawla HM, Singh SP (2007) Calixarenes as sensor materials for recognition and separation of metal ions. ARKIVOC II:172–200
Hascall T, Pang K, Parkin G (2007) Exo and endo isomerism of subvalent tin and germanium complexes derived from 1,3-diethers of p-tert-butylcalix[4]arene. Tetrahedron 63(44):10826–10833
Ali A, Flora SJS, Saxena G, Kolehmainen E, Mahieu B, Rao CP (2006) Synthesis and characterization of Sn(IV) complexes of lower rim 1, 3-diacid derivative of calix[4]arene and their protective effects on tissue oxidative stress and essential metal concentration in lead exposed male Wistar rats. J Inorg Biochem 100(2):167–304
Torgov V, Kostin G, Korda T, Stoyanov E, Kalchenko V, Drapailo A, Kasyan O, Wipff G, Varnek A (2005) Upper rim thioether derivatives of calix[4,6]arenes: extraction of fission Pd(II) and Ag(I). Solv Extract Ion Exchange 23(6):781–801
Makrlik E, Budka J, Vanura P, Selucky P (2010) Extraction of europium trifluoromethanesulfonate into nitrobenzene in the presence of some electroneutral calixarene ligands. J Radioanal Nucl Chem 283(1):193–196
Ayata S, Merdivan M (2010) p-tert-Butylcalix[8]arene loaded silica gel for preconcentration of uranium(VI) via solid phase extraction. J Radioanal Nucl Chem 283(3):603–607
Mohapatra PK, Ansari SA, Sarkar A, Bhattacharyya A, Manchanda VK (2006) Evaluation of calix-crown ionophores for selective separation of radio-cesium from acidic nuclear waste solution. Anal Chim Acta 571(2):308–314
Zhang A, Hu Q (2010) Adsorption of cesium and some typical coexistent elements onto a modified macroporous silica-based supramolecular recognition material. Chem Eng J 159:58–66
Li H, Zhan J, Chen M, Tian D, Zou Z (2010) Metal ions recognition by 1,2,3-triazolium calix[4]arene esters synthesized via click chemistry. J Incl Phenom Macrocycl Chem 66(1–2):43–47
Matulkova I, Rohovec J (2005) Synthesis, characterization and extraction behaviour of calix[4]arene with four propylene phosphonic acid groups on the lower rim. Polyhedron 24:311–317
Mustafina A, Zakharova L, Elistratova J, Kudryashova J, Soloveva S, Garusov A, Antipin I, Konovalov A (2010) Solution behavior of mixed systems based on novel amphiphilic cyclophanes and Triton X100: aggregation, cloud point phenomenon and cloud point extraction of lanthanide ions. J Colloid Interface Sci 346:405–413
Jain VK, Pandya RA, Pillai SG, Shrivastav PS (2006) Simultaneous preconcentration of uranium(VI) and thorium(IV) from aqueous solutions using a chelating calix[4]arene anchored chloromethylated polystyrene solid phase. Talanta 70(2):257–266
Toutianoush A, El-Hashani A, Schnepf J, Tieke B (2005) Multilayer membranes of p-sulfonato-calix[8]arene and polyvinylamine and their use for selective enrichment of rare earth metal ions. Appl Surf Sci 246(4):430–436
Yaftian MR, Razipour MR, Matt D (2006) Extraction of thorium(IV) and europium(III) by a phosphorylated calix[4]arene in dichloromethane. J Radioanal Nucl Chem 270(2):357–361
Sansone F, Fontanella M, Casnati A, Ungaro R, Böhmer V, Saadioui M, Liger K, Dozol JF (2006) CMPO-substituted calix[6]- and calix[8]arene extractants for the separation of An3+/Ln3+ from radioactive waste. Tetrahedron 62(29):6749–6753
Vicens J (2006) Applied and fundamental research: their mutual stimulation in the real world of chemistry-developing calix bis crowns for nuclear waste treatment. J Incl Phenom Macrocycl Chem 55(1–2):193–196
Yang Y, Cao X, Surowiec K, Bartsch RA (2010) Calix[4]arene-thiacrown-5 di(carboxylic acid) regioisomers as metal ion extractants. J Incl Phenom Macrocycl Chem 66(1–2):163–169
Park C, Chun S, Bartsch RA (2010) Effect of conformation on metal ion extraction by calix[4]arene dicarboxylic acids. J Incl Phenom Macrocycl Chem 66(1–2):95–105
Yamato T, Kitajima F, Gil JT (2005) Alkyl ammonium ion selectivity of hexahomotrioxacalix[3]arene triamide derivative having the intramolecular hydrogen-bonding group. J Incl Phenom Macrocycl Chem 53(3):257–262
Li X, Gong SL, Yang WP, Li Y, Chen YY, Meng XG (2010) Aminopyridyl derivative of thiacalix[4]arene-carboxylic acid as ionizable highly selective Ag+ ionophore. J Incl Phenom Macrocycl Chem 66(1–2):179–184
Xia YX, Zhou HH, Yin Y, Qiu N, Luo J, Xiang GY (2010) Intramolecular cyclization strategy: synthesis of 1,3- and 1,2-calix[4]crown-7 and calix[4]crown-9 cone conformers. J Incl Phenom Macrocycl Chem (in press)
Deligoz H, Erdem E (2008) Comparative studies on the solvent extraction of transition metal cations by calixarene, phenol and ester derivatives. J Hazard Mater 154:29–32
Ulewicz M, Lesinska U, Bochenska M, Walkowiak W (2007) Facilitated transport of Zn(II), Cd(II) and Pb(II) ions through polymer inclusion membranes with calix[4]-crown-6 derivatives. Sep Purif Technol 54(3):299–305
Iki N (2009) Non-covalent strategy for activating separation and detection functionality by use of the multifunctional host molecule thiacalixarene. J Incl Phenom Macrocycl Chem 64(1–2):1–13
Kumar A, Sharma P, Chandel LK, Kalal BL (2008) Synergistic extraction and spectrophotometric determination of palladium(II), iron(III), and tellurium(IV) at trace level by newly synthesized p-[4-(3,5-dimethylisoxazolyl)azophenylazo]calix(4)arene. J Incl Phenom Macrocycl Chem 61(3–4):335–342
Ludwig R, Dzunga NTK (2005) Solvent extraction of Tc(VII) by calixarenes bearing pyridino groups. J Nucl Radiochem Sci 6(3):227–231
Meyer R, Jira T (2007) Calixarene HPLC phases—applications. Curr Anal Chem 3(2):161–170
Bouvier-Capely C, Manoury A, Legrand A, Bonthonneau JP, Cuenot F (2009) The use of calix[6]arene molecules for actinides analysis in urine and drinking water: an alternative to current procedures. J Radioanal Nucl Chem 282(2):611–615
Zhang A, Kuraoka E, Kumagai M (2007) Development of the chromatographic partitioning of cesium and strontium utilizing two macroporous silica-based calix[4]arene-crown and amide impregnated polymeric composites: PREC partitioning process. J Chromatogr A 1157(1–2):85–95
Zhang A, Wei Y, Hoshi H, Koma Y, Kamiya M (2007) Partitioning of cesium from a simulated high level liquid waste by extraction chromatography utilizing a macroporous silica-based supramolecular calix[4]arene-crown impregnated polymeric composite. Solv Extract Ion Exchange 25(3):389–405
Gok C, Seyhan S, Merdivan M, Yurdakoc M (2007) Separation and preconcentration of La3+, Ce3+ and Y3+ using calix[4]resorcinarene impregnated on polymeric support. Microchimica Acta 157(1–2):13–19
Zhang A, Kuraoka E, Kumagai M (2006) Removal of Pd(II), Zr(IV), Sr(II), Fe(III), and Mo(VI) from simulated high level liquid waste by extraction chromatography utilizing the macroporous silica-based polymeric materials. Sep Purif Technol 50(1):35–44
Valeur B, Leray I (2007) Ion-responsive supramolecular fluorescent systems based on multichromophoric calixarenes: a review. Inorg Chim Acta 360(3):765–774
Kim JS, Quang DT (2007) Calixarene-derived fluorescent probes. Chem Rev 107:3780–3799
Oueslati I, Ferreira RAS, Carlos LD, Baleizao C, Berberan-Santos MN, Castro B, Vicens J, Pischel U (2006) Calix[4]azacrowns as novel molecular scaffolds for the generation of visible and near-infrared lanthanide luminescence. Inorg Chem 45(6):2652–2660
Leray I, Valeur B (2009) Calixarene-based fluorescent molecular sensors for toxic metals. Eur J Inorg Chem 2009(24):3525–3535
Lee MH, Quang DT, Jung HS, Yoon J, Lee CH, Kim JS (2007) Ion-induced FRET on–off in fluorescent calix[4]arene. J Org Chem 72(11):4242–4245
Ocak Ü, Ocak M, Surowiec K, Bartsch RA, Gorbunova MG, Tu C, Surowiec MA (2009) Metal ion complexation in acetonitrile by di-ionized calix[4]arenes bearing two dansyl fluorophores. J Incl Phenom Macrocycl Chem 63(1–2):131–139
Kim HJ, Kim JS (2006) BODIPY appended cone-calix[4]arene: selective fluorescence changes upon Ca2+ binding. Tetrahedron Lett 47(39):7051–7055
Liu Z, Jiang L, Liang Z, Gao Y (2006) A selective colorimetric chemosensor for lanthanide ions. Tetrahedron 62(14):3214–3220
Liang Z, Liu Z, Gao Y (2007) A selective colorimetric chemosensor based on calixarene framework for lanthanide ions-Dy3+ and Er3+. Tetrahedron Lett 48:3587–3590
El-Nashar RM, Wagdy HAA, Aboul-Enein HY (2009) Applications of calixarenes as potential ionophores for electrochemical sensors. Curr Anal Chem 5(3):249–270
Hassanzadeh P, Yaftian MR, Bahari Z, Matt D (2006) A coated graphite thorium-ion selective potentiometric sensor based on a calix[4]arene bearing phosphoryl groups. J Chin Chem Soc 53:1113–1118
Gupta VK, Ludwig R, Agarwal S (2005) Strontium(II) sensor based on a modified calix[6]arene in PVC matrix. Anal Sci 21(3):293–296
Kim TH, Kim H, Lee JH, Kim JS (2008) Sr2+ ion selective p-tert-butylthiacalix[4]arene bearing two distal amide units. Bull Korean Chem Soc 29(3):620–622
Zheng H, Yan Z, Dong H, Ye B (2007) Simultaneous determination of lead and cadmium at a glassy carbon electrode modified with Langmuir–Blodgett film of p-tert-butylthiacalix[4]arene. Sens Actuators B Chem 120(2):603–609
Dong H, Zheng H, Lin L, Ye B (2006) Determination of thallium and cadmium on a chemically modified electrode with Langmuir–Blodgett film of p-allylcalix[4]arene. Sens Actuators B Chem 115(1):303–308
Becker A, Tobias H, Porat Z, Mandler D (2008) Detection of uranium(VI) in aqueous solution by a calix[6]arene modified electrode. J Electroanal Chem 621(2):214–221
Acknowledgments
This work was supported by National Iranian Oil Company and Islamic Azad University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mokhtari, B., Pourabdollah, K. & Dallali, N. A review of calixarene applications in nuclear industries. J Radioanal Nucl Chem 287, 921–934 (2011). https://doi.org/10.1007/s10967-010-0881-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0881-1