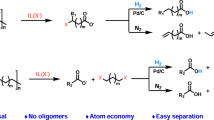
Abstract
A series of chemical recycling methods have been developed for polyamides (PAs) for green and sustainable technologies of plastic waste. However, the study of the essential mechanism of advanced thermo-chemical recycling of PAs is still lacking. To select efficient and green catalysts, a thorough understanding of the limiting factors of PAs hydrolysis reactions is necessary. Herein, to discuss the limiting factors of hydrolysis and the catalytic mechanism of common catalysts, polyamide 4 (PA4), a green and sustainable advanced polyamide, was studied. Firstly, the hydrolysis products and the degradation mechanism of PA4 were investigated. The only product is butyrolactam. Hydrolysis is characterized by both a depolymerization mechanism and a random chain scission mechanism. Secondly, the kinetic process of hydrolysis was investigated from the perspectives of molecular weight and solid product mass (\({\text{E}{\upalpha }}_{\left({\text{M}}_{\text{v}}\right)}=15.87\text{k}\text{J}/\text{m}\text{o}\text{l}, {\text{E}{\upalpha }}_{({\text{R}}_{\text{s}\text{r}}, 1)}=44.87\text{k}\text{J}/\text{m}\text{o}\text{l}, {\text{E}{\upalpha }}_{({\text{R}}_{\text{s}\text{r}}, 2)}=89.71\text{k}\text{J}/\text{m}\text{o}\text{l}\)). Finally, the existence of phase separation near the polymer chains was verified both experimentally and by molecular dynamics simulations. Furthermore, the extremely high correlation (r = 0.95, p < 0.05) between the molecular diffusion rate and the hydrolysis rate under certain reaction conditions explained the catalytic mechanism of common catalysts. Compared with the proton concentration in the system, the mass transfer efficiency in polymer chain cages is a more essential restricting factor. This mechanism is available to all PAs and provides a theoretical support for the selection of green catalysts for polyamide hydrolysis, which will greatly promote the application of chemical recycling technology.








Similar content being viewed by others
Data availability
All relevant data are within the manuscript and its Additional files.
References
Perryman M, Law, Kara, Lavender, Wilcox et al (2015) Plastic waste inputs from land into the ocean. Science 347(62):768–771
Rachman CM (2018) Microplastics research—from sink to source. Science 360(6384):28
Zhang F, Zhao Y, Wang D, Yan M, Chen C (2020) Current technologies for plastic waste treatment: a review. J Clean Prod 282:124523
Kawashima N, Yagi T, Kojima K (2019) How do bioplastics and fossil-based plastics play in a circular economy? Macromol Mater Eng 304(9):1900383
Mondragon G, Kortaberria G, Eider M, Gonzalez N, Pea C (2019) Thermo-mechanical recycling of polyamide 6 from fishing nets waste. J Appl Polym Sci 137(10):1–6
Formisano B, Gttermann S, Bonten C (2016) Recycling of cast polyamide waste on a twin-screw-extruder. PROCEEDINGS OF THE REGIONAL CONFERENCE GRAZ 2015 – POLYMER PROCESSING SOCIETY PPS: Conference Papers
Weinmann S, Bonten C (2020) Recycling of PA12 powder for selective laser sintering. fracture and damage mechanics: theory, simulation and experiment
Al-Salem S, Lettieri P, Baeyens J (2009) Recycling and recovery routes of plastic solid waste (psw): a review. Waste Manag 29(10):2625–2643
Fagnani D, Tami J, Copley G, Clemons M, Mcneil AJ (2020) 100th anniversary of macromolecular science viewpoint: redefining sustainable polymers. ACS Macro Lett 10:41–53
Earek U, Pahovnik D, Agar E (2020) Chemical recycling of aliphatic polyamides by microwave-assisted hydrolysis for efficient monomer recovery. ACS Sustain Chem Eng 8(43):16274–16282
Alberti C, Figueira R, Hofmann M, Koschke S, Enthaler S (2019) Chemical Recycling of End-of‐Life Polyamide 6 via Ring closing depolymerization. Chem Select 4(43):12638–12642
Datta J, Błażek K, Włoch M, Bukowski R et al (2018) A new approach to chemical recycling of polyamide 6.6 and synthesis of polyurethanes with recovered intermediates. J Polym Environ 26(12):4415–4429
Maza S, Lefebvre X, Brusselle-Dupend N, Klopffer M, Grandidier J (2019) Physicochemical and mechanical degradation of polyamide 11 induced by hydrolysis and thermal aging. J Appl Polym Sci 136(11):47628
Nemade A, Mishra S, Zope V (2011) Chemical recycling of polyamide waste at various temperatures and pressures using high pressure autoclave technique. J Polym Environ 19(1):110–114
Goto M (2009) Chemical recycling of plastics using sub- and supercritical fluids. J Supercrit Fluids 47(3):500–507
Wei W, Meng L, Huang Y (2014) Hydrolytic degradation of monomer casting nylon in subcritical water. Polym Degrad Stab 110:312–317
Chen J, Liu G, Jin L, Ni P, Li Z, He H et al (2010) Catalytic hydrolysis of waste nylon 6 to produce s-caprolactam in sub-critical water. J Anal Appl Pyrol 87(1):50–55
Bckstrm E, Odelius K, Hakkarainen M (2021) Microwave assisted selective hydrolysis of polyamides from multicomponent carpet waste. Global Challenges 5(7):2000119
Shukla S, Ajay H, Mahato D (2006) Depolymerization of nylon 6 waste fibers. J Appl Polym Sci 100(1):186–190
Mukherjee A, Goel D (1978) Depolymerization of poly-c-caprolactam catalyzed by sodium hydroxide. J Appl Polym Sci 22:361–368
Czernik S, Elam C, Evans R, Meglen R, Tatsumoto K (1998) Catalytic pyrolysis of nylon-6 to recover caprolactam. J Anal Appl Pyrol 46(1):51–64
Kamimura A, Yamamoto S (2008) A novel depolymerization of nylons in ionic liquids. Polym Adv Technol 19(10):1391–1395
Kamimura A, Shiramatsu Y, Kawamoto T (2019) Depolymerization of polyamide 6 in hydrophilic ionic liquids. Green Energy & Environment 4(2):166–170
Khuntia S, Gadgeel A, Mestry S, Mhaske S (2022) Organo-sulfonic acid catalyzed degradation kinetics and thermodynamic studies of nylon‐6 by hydrothermal method. Polym Adv Technol 33(1):411–426
KFJ Schoch (1996) Nylon plastics handbook. Electr Insul Magazine IEEE 12(5):48
Chaupart N, Serpe G, Verdu J (1998) Molecular weight distribution and mass changes during polyamide hydrolysis. Polymer 39(6–7):1375–1380
Qda B, Mlg A, Cd B et al (2021) Modelling pure polyamide 6 hydrolysis: influence of water content in the amorphous phase. Polym Degrad Stab 183:1–8
Jacques B, Werth M, Merdas I, Thominette F, Verdu J (2002) Hydrolytic ageing of polyamide 11. 1. Hydrolysis kinetics in water. Polymer 43(24):6439–6447
Hopewell J, Dvorak R, Kosior E (2009) Plastics recycling: challenges and opportunities. Philosophical Trans Royal Soc B Biol Sci 364(1526):2115–2126
Lin, Gao L, Guangxian (2015) Long-term hydrothermal aging behavior and life-time prediction of polyamide 6. J Macromolecular Sci Phys 54(3):239–252
Sierra A, Alper H (2020) Progress in the metabolic engineering of bio-based lactams and their ω-amino acids precursors. Biotechnol Adv 43:107587
Park S, Kim E, Noh W, Oh Y, Kim H, Song B et al (2013) Synthesis of nylon 4 from gamma-aminobutyrate (gaba) produced by recombinant escherichia coli. Bioprocess & Biosystems Engineering 36(7):885–892
Yamano N, Kawasaki N, Takeda S, Nakayama A (2013) Production of 2-pyrrolidone from biobased glutamate by using escherichia coli. J Polym Environ 21(2):528–533
Zhang J, Kao E, Wang G, Baidoo E, Chen M, Keasling J (2016) Metabolic engineering of escherichia coli for the biosynthesis of 2-pyrrolidone. Metabolic Eng Commun 3:1–7
Tachibana K, Hashimoto K, Yoshikawa M, Okawa H (2010) Isolation and characterization of microorganisms degrading nylon 4 in the composted soil. Polym Degrad Stab 95(6):912–917
Naoko Y, Atsuyoshi N, Norioki, Kawasaki et al (2008) Mechanism and characterization of polyamide 4 degradation by pseudomonas sp. J Polym Environ 16(2):141–146
Yamano N, Kawasaki N, Oshima M, Nakayama A (2014) Polyamide 4 with long-chain fatty acid groups - suppressing the biodegradability of biodegradable polymers. Polym Degrad Stab 108:116–122
Sasanami Y, Honda M, Nishiki H, Tachibana K, Abe H, Hokamura A et al (2022) Purification and characterization of an enzyme that degrades polyamide 4 into gamma-aminobutyric acid oligomers from pseudoxanthomonas sp. TN-N1. Polym Degrad Stab 197:1–8
Tachibana K, Urano Y, Numata K (2013) Biodegradability of nylon 4 film in a marine environment. Polym Degrad Stab 98(9):1847–1851
Yamano N, Kawasaki N, Ida S, Nakayama Y, Nakayama A (2017) Biodegradation of polyamide 4 in vivo. Polym Degrad Stab 137:281–288
Norioki K, Atsuyoshi N, Naoko et al (2005) Synthesis, thermal and mechanical properties and biodegradation of branched polyamide 4. Polymer 46(23):9987–9993
Kim N, Kim J, Nam S, Jeon B, Yoo Y, Kim Y et al (2013) Synthesis and characterization of very high molecular weight nylon 4 and nylon 4/6 copolymers. Polym Korea 37(2):211–217
Dencheva N, Braz J, Nunes, Teresa et al (2018) One-pot low temperature synthesis and characterization of hybrid poly(2-pyrrolidone) microparticles suitable for protein immobilization. Polymer: The International Journal for the Science and Technology of Polymers 145:402–415
Dencheva N, Braz J, Scheibel D, Malfois M, Gitsov I (2020) Polymer-assisted biocatalysis: polyamide 4 microparticles as promising carriers of enzymatic function. Catalysts 10(7):767
Wen-jing H, Tao C, Li-ming Z, Jie W, Yong-jun Q, Jia-wei L (2017) Nonisothermal crystallization kinetics of poly(2-pyrrolidone). J Funct Polym 30(3):314–320
Basconi J, Shirts M (2013) Effects of temperature control algorithms on transport properties and kinetics in molecular dynamics simulations. J Chem Theory Comput 9(7):2887–2899
Huai S, Zhao, Jin C, Yang et al (2016) Compass ii: extended coverage for polymer and drug-like molecule databases. J Mol Model 22(2):47
Evans G, Lesser A (2018) Processing polyamides with superheated water. J Polym Sci Part B: Polym Phys 56(10):803–813
Vinken E, Terry A, Asselen O, Spoelstra A, Rastogi S (2008) Role of superheated water in the dissolution and perturbation of hydrogen bonding in the crystalline lattice of polyamide 4,6. Langmuir the Acs Journal of Surfaces & Colloids 24(12):6313–6326
Schroeder LR, Cooper SL (1976) Hydrogen bonding in polyamides. J Appl Phys 47(2):4310
Fredericks R, Doyne T, Sprague R (1966) Crystallographic studies of nylon 4. i. determination of the crystal structure of the α polymorph of nylon 4. J Polym Sci Part A-2 Polym Phys 4(6):899–911
Costa D, Costa M, Grytten F (2022) Morphological changes of polyamide 11 through the corrected inherent viscosity plateau. J Appl Polym Sci 139(21):1–8
Hocker S, Rhudy A, Ginsburg G, Kranbuehl D (2014) Polyamide hydrolysis accelerated by small weak organic acids. Polymer 55(20):5057–5064
Ohno M, Manalo C, Rossetto L, Laura et al (2016) Effect of coexisting metal ions on the degradation of polyamide reverse osmosis membrane by hypochlorite treatment. Desalination: The International Journal on the Science and Technology of Desalting and Water Purification 381:126–134
Hashiba K, Nakai S, Nishijima W, Ohno M, Gotoh T (2020) Degradation of secondary polyamide reverse osmosis membrane by hypochlorite in the presence of calcium ions. Polym Degrad Stab 181:1–7
Do V, Tang C, Reinhard M, Leckie J (2012) Effects of chlorine exposure conditions on physiochemical properties and performance of a polyamide membrane—mechanisms and implications. Environ Sci Technol 46(24):13184–13192
Klun U, Krzan A (2002) Degradation of polyamide-6 by using metal salts as catalyst. Polym Adv Technol 13(10–12):817–822
Gagliardi M (2019) Mathematical modeling and experimental study of water diffusion and swelling in polymer films. Macromol Ther Simul 28:1800063
Philip A, Gale, Sergio E, García-Garrido et al (2008) Anion receptors based on organic frameworks: highlights from 2005 and 2006. Chem Soc Rev 37(1):151–190
Xie L, He X, Liu Y, Cao C, Zhang W (2022) Treatment of reverse osmosis membrane by sodium hypochlorite and alcohols for enhanced performance using the swelling-fastening effect. Chemosphere 292:133444
Klun U, Kran A (2000) Rapid microwave induced depolymerization of polyamide-6. Polymer 41(11):4361–4365
Bockhorn H, Hornung A, Hornung U, Weichmann J (1999) Kinetic study on the non-catalysed and catalysed degradation of polyamide 6 with isothermal and dynamic methods. Thermochimica acta 337(1):97–110
Philip M, Hong G (2009) Corrosion control methods in supercritical water oxidation and gasification processes. J Supercrit Fluids 51(2):83–103
Funding
This work was supported by the Research and Development of Technical Standards for Bio-based Material Polybutyrolactam Project (21DZ2205900), the National Key R&D Program of China (2022YFC2104500), the 111 Project (B18022), the Fundamental Research Funds for the Central Universities (22221818014), and the Open Project Funding of the State Key Laboratory of Bioreactor Engineering, ECUST (ZDXM2019), and Shanghai Sailing Program (20YF1409900).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Qiu, Y., Chen, T. et al. The catalytic mechanism and limiting factor of polyamide hydrolysis for chemical recycling: the classic hydrolysis of polyamide 4. J Polym Res 30, 186 (2023). https://doi.org/10.1007/s10965-023-03567-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03567-z