Abstract
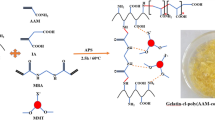
In this study, the hydrogels were synthesized by grafting copolymers of methacrylic acid (MAA) and acrylamide (AAm) onto the sodium alginate (SA) backbone by free radical polymerization method to remove malachite green (MG) from aqueous solution. Montmorillonite (MMT) clay nanoparticles with different ratios (0, 5, 10, and 15 wt.%) were added to the hydrogel to improve the swelling and MG adsorption behavior of the SA-g-poly(MMA-co-AAm)/MMT nanocomposite hydrogels. The optimum weight percentage of nanoparticles was obtained at 10 wt.% based on the SA-g-poly(MMA-co-AAm)/MMT nanocomposite hydrogel adsorption performance. The prepared adsorbents were characterized by FTIR, SEM, XRD, TGA, and EDX analysis. The results revealed that the SA-g-poly(MMA-co-AAm)/MMT nanocomposite hydrogel was successfully prepared. The maximum adsorption capacity of 95.91 mg/g for the adsorbent was obtained at a pH of 7, an adsorbent dosage of 1.5 g/L, a temperature of 25 °C, a contact time of 90 min, and MG concentration of 10 mg/L. The isotherm and kinetics results showed that the MG adsorption process followed the Langmuir and pseudo-second-order adsorption models. In thermodynamics studies, a negative value of Gibbs free energy (ΔG°) indicated the spontaneous nature of the adsorption process. In addition, SA-g-poly(MMA-co-AAm) and SA-g-poly(MMA-co-AAm)/MMT nanocomposite hydrogels gave adsorption enthalpies (ΔH°) of -61.512 and -77.281 kJ/mol, respectively, which proved the exothermic nature of the MG adsorption when using these adsorbents.









Similar content being viewed by others
References
Li Y et al (2020) Redox-responsive carboxymethyl cellulose hydrogel for adsorption and controlled release of dye. Eur Polymer J 123:109447
Xiao D et al (2020) Strong alginate/reduced graphene oxide composite hydrogels with enhanced dye adsorption performance. Polym Bull 77(12):6609–6623
Godiya CB, Kumar S, Xiao Y (2020) Amine functionalized egg albumin hydrogel with enhanced adsorption potential for diclofenac sodium in water. J Hazard Mater 393:122417
Mok CF et al (2020) Adsorption of dyes using poly (vinyl alcohol)(PVA) and PVA-based polymer composite adsorbents: a review. J Polym Environ 28(3):775–793
Tabari MA et al (2021) Antitrichomonal activity of metronidazole-loaded lactoferrin nanoparticles in pigeon trichomoniasis. Parasitol Res 120:3263–3272
Abatari MN et al (2017) Superporous pellicular κ-Carrageenan–Nickel composite beads; morphological, physical and hydrodynamics evaluation for expanded bed adsorption application. Chem Eng Res Des 125:291–305
Al-Aidy, H, Amdeha E (2021) Green adsorbents based on polyacrylic acid-acrylamide grafted starch hydrogels: the new approach for enhanced adsorption of malachite green dye from aqueous solution. Int J Environ Anal Chem 101(15):2796–2816
Khalili M et al (2022) High durability of food due to the flow cytometry proved antibacterial and antifouling properties of TiO2 decorated nanocomposite films. Food Chem Toxicol 168:113291
Lashkenari AS et al (2019) Direct filtration procedure to attain antibacterial TFC membrane: A facile developing route of membrane surface properties and fouling resistance. Chem Eng Res Des 149:158–168
Mashhadzadeh AH et al (2018) DFT study of Ni, Cu, Cd and Ag heavy metal atom adsorption onto the surface of the zinc-oxide nanotube and zinc-oxide graphene-like structure. Mater Chem Phys 220:366–373
Pauletto P et al (2020) Single and competitive dye adsorption onto chitosan–based hybrid hydrogels using artificial neural network modeling. J Colloid Interface Sci 560:722–729
Rad AS et al (2021) Are nickel- and titanium- doped fullerenes suitable adsorbents for dopamine in an aqueous solution? Detailed DFT and AIM studies. J Mol Liq 322:114942
Abdolahi G, Dargahi M, Ghasemzadeh H (2020) Synthesis of starch-g-poly (acrylic acid)/ZnSe quantum dot nanocomposite hydrogel, for effective dye adsorption and photocatalytic degradation: thermodynamic and kinetic studies. Cellulose 27:6467–6483
Emadian SM et al (2015) Treatment of low-strength bilge water of Caspian Sea ships by HUASB technique. Ecol Eng 82:272–275
Hosseini M, Shahavi MH, Yakhkeshi A (2012) AC & DC-currents for separation of nano-particles by external electric field. Asian J Chem 24(1):181–184
Hosseini M, Shahavi MH (2012) Electrostatic enhancement of coalescence of oil droplets (in nanometer scale) in water emulsion. Chin J Chem Eng 20(4):654–658
Mofidian R et al (2020) Generation process and performance evaluation of engineered microsphere agarose adsorbent for application in fluidized-bed systems. Int J Eng 33(8):1450–1458
Mofidian R et al (2020) Fabrication of novel agarose–nickel bilayer composite for purification of protein nanoparticles in expanded bed adsorption column. Chem Eng Res Des 159:291–299
Nayak S et al (2020) Carbon dot cross-linked polyvinylpyrrolidone hybrid hydrogel for simultaneous dye adsorption, photodegradation and bacterial elimination from waste water. J Hazard Mater 392:122287
Selakjani PP et al (2021) Reducing free formaldehyde emission, improvement of thickness swelling and increasing storage stability of novel medium density fiberboard by urea-formaldehyde adhesive modified by phenol derivatives. Int J Adhes Adhes 111:102962
Das L et al (2020) Synthesis of hybrid hydrogel nano-polymer composite using Graphene oxide, Chitosan, and PVA and its application in wastewater treatment. Environ Technol Innov 18:100664
Ghanbarzadeh, M., et al., (2022) Removal of Phenanthrene by some microalga species and study of antioxidative compounds in Nostoc calcicola ISC 89. Journal of Soils and Sediments 22: p. 109-119.
Yu QJ et al (2020) A simple multifunctional PNIPAM-GO/PANI hydrogel preparation strategy and its application in dye adsorption and infrared switching. J Macromol Sci Part A 57(11):751–760
Kazemeini H, Azizian A, Shahavi MH (2019) Effect of Chitosan Nano-Gel/Emulsion containing Bunium Persicum essential oil and Nisin as an edible biodegradable coating on Escherichia Coli O157:H7 in rainbow trout fillet. J Water Environ Nanotechnol 4(4):343–349
Rajalekshmy G, Rekha M (2021) Strontium ion cross-linked alginate-g-poly (PEGMA) xerogels for wound healing applications: in vitro studies. Carbohyd Polym 251:117119
Verma A et al (2020) Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int J Biol Macromol 148:1130–1139
Ilić-Stojanović S et al (2021) Semi-Crystalline copolymer hydrogels as smart drug carriers: in vitro thermo-responsive naproxen release study. Pharmaceutics 13(2):158
Yeo YH, Park WH (2021) Dual-crosslinked, self-healing and thermo-responsive methylcellulose/chitosan oligomer copolymer hydrogels. Carbohyd Polym 258:117705
Carleton MM, Locke M, Sefton MV (2021) Methacrylic acid-based hydrogels enhance skeletal muscle regeneration after volumetric muscle loss in mice. Biomaterials 275:120909
Liu Y et al (2021) Novel graphene oxide Nanohybrid doped Methacrylic acid hydrogels for enhanced swelling capability and cationic adsorbability. Polymers 13(7):1112
Sabbagh F (2021) A comparative study on the clays incorporated with acrylamide-based hydrogels. Adv Appl NanoBio-Technol 2(4):15–23
Tirtom VN, Dinçer A (2021) Effective removal of heavy metals from an aqueous solution with poly (N-vinylimidazole-acrylamide) hydrogels. Sep Sci Technol 56(5):912–924
MohammadzadehPakdel P, Peighambardoust SJ, Arsalani N, Aghdasinia H (2022) Safranin-O cationic dye removal from wastewater using carboxymethyl cellulose-grafted-poly (acrylic acid-co-itaconic acid) nanocomposite hydrogel. Environ Res 212:113201
Safarzadeh H, Peighambardoust SJ, Mousavi SH, Foroutan R, Mohammadi R, Peighambardoust SH (2022) Adsorption ability evaluation of the poly (methacrylic acid-co-acrylamide)/cloisite 30B nanocomposite hydrogel as a new adsorbent for cationic dye removal. Environ Res 212:113349
Safarzadeh H, Peighambardoust SJ, Mousavi SH, Mohammadi R, Peighambardoust SH (2022) Adsorption of methyl violet dye from wastewater using poly(methacrylic acid-co-acrylamide)/bentonite nanocomposite hydrogels. J Polym Res 29(4):113
Kenawy ER, Azaam MM, El-nshar EM (2019) Sodium alginate-g-poly (acrylic acid-co-2-hydroxyethyl methacrylate)/montmorillonite superabsorbent composite: preparation, swelling investigation and its application as a slow-release fertilizer. Arab J Chem 12(6):847–856
Peighambardoust SJ et al (2020) Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int J Biol Macromol 159:1122–1131
Sarkar N, Sahoo G, Swain SK (2020) Nanoclay sandwiched reduced graphene oxide filled macroporous polyacrylamide-agar hybrid hydrogel as an adsorbent for dye decontamination. Nano-Struct Nano-Objects 23:100507
Shahbazi M et al (2020) Electron beam crosslinking of alginate/nanoclay ink to improve functional properties of 3D printed hydrogel for removing heavy metal ions. Carbohyd Polym 240:116211
Mittal H et al (2020) In-situ synthesis of ZnO nanoparticles using gum arabic based hydrogels as a self-template for effective malachite green dye adsorption. J Polym Environ 28(6):1637–1653
Rabipour M et al (2020) pH-sensitive nanocomposite hydrogels based on poly (vinyl alcohol) macromonomer and graphene oxide for removal of cationic dyes from aqueous solutions. J Polym Environ 28(2):584–597
Weerasundara L et al (2021) Hydrogels: Novel materials for contaminant removal in water—A review. Crit Rev Environ Sci Technol 15(17):1970–2014
Abdolhosseinzadeh M et al (2018) Swelling and auramine-O adsorption of carboxymethyl cellulose grafted poly (methyl methacrylate)/Cloisite 30B nanocomposite hydrogels. Iran Polym J 27(10):807–818
Ibrahim AG et al (2020) Synthesis of a hydrogel by grafting of acrylamide-co-sodium methacrylate onto chitosan for effective adsorption of Fuchsin basic dye. Int J Biol Macromol 159:422–432
Mate CJ, Mishra S, Srivastava P (2021) Design of pH sensitive low-cost adsorbent from the exudate of Lannea coromandelica (Houtt) for remediation of Malachite Green dye from aqueous solution. Polym Bull 78(7):3459–3487
Pourjavadi A, Doulabi M, Doroudian M (2014) Adsorption characteristics of malachite green dye onto novel kappa-carrageenan-g-polyacrylic acid/TiO2–NH2 hydrogel nanocomposite. J Iran Chem Soc 11(4):1057–1065
Rahimi K et al (2018) Preparation of nanoparticle-modified polymeric adsorbent using wastage fuzzes of mechanized carpet and its application in dye removal from aqueous solution. J Clean Prod 178:373–383
Rajput S, Pittman CU Jr, Mohan D (2016) Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J Colloid Interface Sci 468:334–346
Sharma K et al (2015) Synthesis, characterization and water retention study of biodegradable Gum ghatti-poly (acrylic acid–aniline) hydrogels. Polym Degrad Stab 111:20–31
Zheng Y, Zhu Y, Wang A (2014) Highly efficient and selective adsorption of malachite green onto granular composite hydrogel. Chem Eng J 257:66–73
Hayati-Ashtiani M (2012) Use of FTIR spectroscopy in the characterization of natural and treated nanostructured bentonites (montmorillonites). Part Sci Technol 30(6):553–564
Koosha M, Hamedi S (2019) Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog Org Coat 127:338–347
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022a) Cadmium ion removal from aqueous media using banana peel biochar/Fe3O4/ZIF-67. Environ Res 211:113020
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022b) Development of new magnetic adsorbent of walnut shell ash/starch/Fe3O4 for effective copper ions removal: Treatment of groundwater samples. Chemosphere 296:133978
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022c) Application of walnut shell ash/ZnO/K2CO3 as a new composite catalyst for biodiesel generation from Moringa Oleifera oil. Fuel 311:122624
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022d) Application of waste chalk/CoFe2O4/K2CO3 composite as a reclaimable catalyst for biodiesel generation from sunflower oi”. Chemosphere 289:133226
Foroutan R, Peighambardoust SJ, Mohammadi R, Peighambardoust SH, Ramavandi B (2022e) Generation of biodiesel from edible waste oil using ZIF-67-KOH modified Luffa Cylindrica biomass catalyst. Fuel 322:124181
Ahmadi A, Foroutan R, Esmaeili H, Peighambardoust SJ, Hemmati S, Ramavandi B (2022) Montmorillonite clay/starch/CoFe2O4 nanocomposite as a superior functional material for uptake of cationic dye molecules from water and wastewater. Mater Chem Phys 284:126088
Peighambardoust SJ et al (2022) Sono-photocatalytic activity of sea sediment@400/ZnO catalyst to remove cationic dyes from wastewater. J Mol Liq 367:120478
Khatooni H et al (2023) Adsorption of methylene blue using sodium carboxymethyl cellulose-g-poly(acrylamide-co-methacrylic acid)/Cloisite 30B nanocomposite hydrogel. J Polym Environ 31(1):297–311
Agnihotri S, Singhal R (2019) Effect of sodium alginate content in acrylic acid/sodium humate/sodium alginate superabsorbent hydrogel on removal capacity of MB and CV dye by adsorption. J Polym Environ 27(2):372–385
Junlapong K et al (2020) Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel. Int J Biol Macromol 158:258–264
Ahmadi A et al (2020) The role of bentonite clay and bentonite clay@ MnFe2O4 composite and their physico-chemical properties on the removal of Cr (III) and Cr (VI) from aqueous media. Environ Sci Pollut Res :1–14
Wu Q et al (2019) One-step synthesis of Cu (II) metal–organic gel as recyclable material for rapid, efficient and size selective cationic dyes adsorption. J Environ Sci 86:203–212
Su T et al (2020) Incorporation of dumbbell-shaped and Y-shaped cross-linkers in adjustable pullulan/polydopamine hydrogels for selective adsorption of cationic dyes. Environ Res 182:109010
Sabbagh F et al (2019) Mechanical properties and swelling behavior of acrylamide hydrogels using montmorillonite and kaolinite as clays. J Environ Treat Tech 7(2):211–219
Fan T et al (2008) Biosorption of cadmium (II), zinc (II) and lead (II) by Penicillium simplicissimum: Isotherms, kinetics, and thermodynamics. J Hazard Mater 160(2–3):655–661
Ai L et al (2010) Activated carbon/CoFe2O4 composites: facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem Eng J 156(2):243–249
Olad A, Eslamzadeh M, Mirmohseni A (2019) Ion crosslinked poly (acrylic acid-co-acrylamide)/poly (vinyl alcohol)/Cloisite 15 A nanocomposite hydrogels as potential wound dressing films: Effect of clay content on water absorption kinetic and mechanical properties. Polym Compos 40(5):1762–1773
Yao S et al (2017) An anionic metal–organic framework with ternary building units for rapid and selective adsorption of dyes. Dalton Trans 46(10):3332–3337
Chen X et al (2017) Effect of embedded sodium polyacrylate chains on the adsorption mechanism of neutral red by magnetic particles. Chem Eng Res Des 127:223–235
Kumar V, Rehani V, Kaith BS (2018) Synthesis of a biodegradable interpenetrating polymer network of Av-cl-poly (AA-ipn-AAm) for malachite green dye removal: kinetics and thermodynamic studies. RSC Adv 8(73):41920–41937
Sari A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on aluminum biosorption from aqueous solution by brown algae (Padina pavonica) biomass. J Hazard Mater 171(1–3):973–979
Foroutan R et al (2016) Kinetic and equilibrium studies on the adsorption of lead by the chitin of pink shrimp (Solenocera melantho). Entomol Appl Sci Lett 3:20–26
Ranjbari S et al (2020) Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: mechanism, kinetic, isotherms and thermodynamic study. Int J Biol Macromol 155:421–429
Funding
The authors declare no conflict of interest as this research was conducted in the absence of any commercial or financial relationships. This makes this research not interpreted as a possible conflict of interest.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. Hamid Safarzadeh performed the synthesis of experimental samples and collected test data; Seyed Jamaleddin Peighambardoust designed the study and interpreted the results; Seyed Hadi Peighambardoust revised the manuscript critically for important intellectual content and advised on conceptualization. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Consent to participate and publish
All authors of this manuscript have declared their consent to participate and publish this paper.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Safarzadeh, H., Peighambardoust, S.J. & Peighambardoust, S.H. Application of a novel sodium alginate-graft-poly(methacrylic acid-co-acrylamide)/montmorillonite nanocomposite hydrogel for removal of malachite green from wastewater. J Polym Res 30, 157 (2023). https://doi.org/10.1007/s10965-023-03531-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03531-x