Abstract
In the current study, a hydroxylated chalcone (1-phenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one, 4-aminobenzoic acid, and paraformaldehyde were combined in ethanol/toluene solvent to form a new benzoxazine monomer. 1H NMR and FTIR analysis were used to confirm the produced monomer. Polybenzoxazine was prepared by the thermal curing of chalcone-based benzoxazine monomer and examined using FTIR and XRD. Magnetite nanoparticles were prepared using two different solvents and mixed with benzoxazine monomer at various ratios, followed by insitu thermal curing to prepare polybenzoxazine/magnetite nanocomposites. These nanocomposites were analyzed by FTIR, XRD, DSC and TGA were used to examine the thermal characteristics of the resulted materials. The surface morphology was investigated using SEM, and the magnetic property was measured by VSM. The resulting benzoxazine monomer has a low curing temperature (160 °C). The addition of nanoparticles of magnetite to benzoxazine improved its thermal stability. Also, the inclusion procedure of magnetite into benzoxazine has a significant impact on its saturation magnetization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A novel kind of phenolic resin known as polybenzoxazine (PBz) has attracted significant interest because of its numerous beneficial characteristics shared with traditional phenolics, including strong heat and chemical resistance, flame retardance, outstanding mechanical capabilities, and a comparatively low cost. In addition, they have other appealing qualities as low water absorption and a near-zero shrinkage cure [1]. The drawbacks of conventional phenolic resins, such as the release of condensation by products and the need to acid or base catalysts for polymerization, have been addressed by the development of polybenzoxazines. Benzoxazines are suitable for a variety of uses, including brake pad adhesives, fire retardants, printed circuit board fiber reinforcement, packaging in the microelectronics industry, and composite materials for the aerospace and construction industries [2,3,4]. However, the conventional polybenzoxazines have some drawbacks, such as the high temperature required for the curing process and the brittleness of the cured materials. Polybenzoxazine’s brittleness was typically caused by various types of chain end groups, which prevented the chain from propagating [5]. Numerous investigations have been carried out to enhance the characteristics of polybenzoxazines and broaden their uses. The studies of polybenzoxazines were directed in two categories: the first is to understand more about the chemistry of polybenzoxazines and the relationship between its structures and the related properties [6,7,8]; the second aims to further increase the material’s qualities as a high-performance material by adding new functional groups in the structure of monomers or by preparation of blends and composites [9,10,11,12]. Benzoxazine monomer can easily be obtained through Mannich reaction between amines, phenols, and formaldehyde [13]. To achieve a variety of molecular design flexibility, the initial phenols and amines can be changed. To enhance the properties of polybenzoxazine, it was prepared using hydroxylated chalcones instead of traditional phenols. From chalcone-containing bisphenol, Lin et al. [14] produced benzoxazine monomer using 1,3-bis(4-hydroxyphenyl) propanone, aniline, and paraformaldehyde. Two procedures were used to cure benzoxazine monomer; thermal and photo-curing. Lin et al. [15] used Mannich condensation to create chalcone-based-benzoxazine monomer from bis(4-hydroxybenzylidene)acetone, aniline, and paraformaldehyde by solvent method. The resulted benzoxazine monomer was polymerized by thermal curing and has a high glass transition temperature Tg (314 °C) and a high char yield of 60% at 800 °C. Another benzoxazine containing chalcone was produced by Muthukkarupan et al. [16] using 1,3-bis(4-hydroxyphenyl)prop-2-en-1-one, aniline, and paraformaldehyde. It was found that; the chalcone-based benzoxazine’s curing temperature was 205 °C, its maximum degradation temperature was 474 °C, with a char yield of 43%, and Tg was noticed at 234 °C. The resulting bezoxazine was blended with bismaleimides, including those based on 4,4′-diaminodiphenylsulfone and 4,4′-diaminodiphenyl-methane, resulting in improvement of the thermal properties by increasing the crosslinking density.
Magnetic nanoparticles (MNPs) have attracted great interest due to their properties of low toxicity, biocompatibility, and super-paramagnetic behavior. MNPs have potential uses including; biomedical applications (drug delivery system, cancer therapy, biosensors, and contract agents for magnetic resonance imaging MRI), environmental uses such cleaning up contaminated soil and ground water, catalysis, and high-density magnetic storage media [17,18,19,20,21]. By employing functional groups like carboxylic acids, phosphates, and sulfates that may bond to the surface of the polymer matrix, magnetite nanoparticles can be effectively distributed [22]. Multi-walled carbon nanotubes/nickel-zinc-ferrite composites were successfully fabricated using the chemical vapor deposition method as mentioned by Mustaffa et al. [23]. Due to their excellent dielectric, thermal stability, and magnetic properties, the resulting nanocomposites were more appropriate for microwave absorping applications. Tanasa et al. [24] prepared hydrogels nanocomposites based on magnetite and polyacrylamide in different ratios. To be more compatible to react with acrylamide monomer, magnetite nanoparticles were functionalized with 3-trimethoxysilylpropyl-methacrylate. Because of their good mechanical and magnetic properties, the obtained nanocomposites have a wide range of applications in the field of soft tissue engineering. Barrera et al. [25] have been reviewed the different magnetic properties of several nanoparticles composed of different transition metals or compounds. It has been showed that the magnetic behavior of these materials critically depends on the size of nanoparticles, distribution, degree of aggregations, and the interactions of magnetic nanoparticles with the given matrix. Magnetic solid-phase extraction and quantification of chromium ions were performed using magnetic nanoparticles as reported by Filik and Avan [26].
The current work focused on; (1) the use of hydroxylated chalcone rather than conventional phenols in the synthesis of a benzoxazine with a chalcone basis; (2) preparation of polybenzoxazine nanocomposites using magnetite nanoparticles at different weight ratios; (3) study of the effect of adding magnetite on the thermal properties of a chalcone containing polybenzoxazine; (4) testing the effect of magnetite preparation conditions on the magnetization of polybenzoxazine/magnetite nanocomposites.
Experimental
Chemicals
4-hydroxybenzaldehyde (LOBA-Chemie, India), acetophenone (ADWIC-Egypt), sodium hydroxide (Bio-Chem for laboratory fine chemicals), paraformaldehyde (Merck, Germany), 4-aminobenzoic acid, ferric chloride hexahydrate, ferrous sulfate heptahydrate, hydrochloric acid (ADWIC-Egypt), absolute ethyl alcohol (Bio.Chem), toluene (NaTco. Lab. chemicals Rea.), dioxane (SD Fine Chem limited-India), diethyl ether (SD Fine-Chem limited-India), oleic acid (Qualikems-India) were purchased. All chemicals were used without further purification.
Preparation of 1-(phenyl)-3(4-hydroxy phenyl)prop-2-en-1-one (ch)
Chalcone (ch) was synthesized according to our previously reported method [27].
Preparation of benzoxazine monomer (Bz) and polybenzoxazine (PBz)
In 250 ml round flask, 4-amino benzoic acid (1.37 g, 10 mmol), and paraformaldehyde (0.6 g, 20 mmol) were dissolved in 40 ml cosolvent of ethanol-toluene (1:1) (V/V) for 30 min. Then, 1-(phenyl)-3(4-hydroxyphenyl)prop-2-en-1-one (ch) (2.24 g, 10 mmol) was added, and the mixture was refluxed at 90 °C while being stirred for 48 h. The reaction was then cooled to room temperature, the precipitate was dissolved in diethyl ether, and washed with a 3 × 50 ml solution of an aqueous basic solution (NaOH 1 M) to give 2 g of Bz monomer. After that, benzoxazine monomer was thermally polymerized by heating for two hours in an oven at 230 °C to give (PBz). Bz monomer and the polymer PBz were synthesized according to Scheme 1.
Preparation of magnetite nanoparticles (MNP)
The preparation of magnetite was according to a previously reported work [28].
Synthesis of polybenzoxazine/magnetite nanocomposites (PBz/MNP) and (PBz/MNP-Amm)
Under the influence of N2 gas, 0.425 g of benzoxazine monomer (Bz) was dissolved in 15 ml of 1,4-dioxane, and then 0.075 g of magnetite nanoparticle was introduced to the benzoxazine monomer solution. 2 ml of oleic acid was added, and the reaction mixture was sonicated for 30 min at 50 °C, then the solvent was evaporated at 70 °C. Thermal curing of the final product took place at 230 °C for two hours to produce (PBz/MNP-15). Different quantities of magnetite (0.025, 0.050 g) and benzoxazine monomer (0.475, 0.45 g) were used following the same operation to afford (PBz/MNP-5) and (PBz/MNP-10) respectively. In addition, for the preparation of (PBz/MNP-Amm) nanocomposites, the same procedures were followed by dispersing magnetite nanoparticles (0.075, 0.025 and 0.050 g) in 5 ml of ammonium hydroxide (NH4OH 30%) and mixed with benzoxazine monomer (0.425, 0.475, and 0.45 g) dissolved in dioxane. Thermal curing of the final products took place at 230 °C for two hours to produce (PBz/MNP-15-Amm), (PBz/MNP-10-Amm), and (PBz/MNP-5-Amm) respectively. Each sample underwent gradual thermal curing at the following temperatures: 120 °C for 1 h, 150 °C for 1 h, 180 °C for 1 h, 200 °C for 1 h, and finally 230 °C for 2 h.
Characterization
FTIR spectra were measured using a Perkin Elmer 1420 spectrometer, whose frequency ranges between 4000 and 400 cm− 1. The GRN,APD 2000 PROXRD was used to measure the XRD data, and it was outfitted with a Ni-filtered Cu-Ka radiation (λ = 1.5418 Å) at a scanning rate of 0.05° per second and a diverging slit of 0.3°. Using CDCl3 as a solvent, the proton nuclear magnetic resonance (1 H NMR) spectra was given by a Bruker at 400 MHz. A Shimadzu-60 differential scanning calorimetry (DSC) was used to examine the curing behavior. DSC scans were performed in a nitrogen atmosphere at a heating rate of 10 °C/min and a gas flow of 20 ml/min. Using a Shimadzu TGA-50 H thermal analyzer system with a flow rate of 40 ml/min, thermal gravimetric analysis (TGA) was performed at temperatures between 30 and 800 °C under N2 gas. A scanning electron microscope (SEM) was used to analyze the surface morphology of nanocomposites (JSM-6400 JEOL). A vibrating sample magnetometer (VSM) with a magnetic field up to 10 kOe at room temperature was used to measure the magnetic characteristics of the samples.
Results and discussion
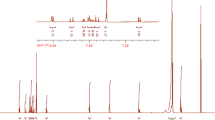
The 1H NMR spectrum of benzoxazine monomer (Bz) is shown in Fig. 1. The characteristic peaks of 1H NMR were as follow; (400 MHz, CDCl3) δ 4.74 (s, 1 H), 4.80 (s, 1 H), 4.89 (s,2 H), 6.89 (d, 1 H), 7.18 (d, 1 H), 7.43 (d, 2 H), 7.50 (d, 2 H), 7.55 (d, 3 H), 7.79 (d, 2 H), 8.02 (d, 2 H), 9.73 (s, 1 H) ppm.
The FTIR analysis of chalcone (ch), benzoxazine monomer (Bz), polybenzoxazine (PBz), magnetite (MNP), and polybenzoxazine/magnetite nanocomposites using different MNP contents is shown in Figs. 2, 3 and 4. For chalcone Ch, the absorption characteristic peaks were recorded at 1650 cm− 1 (C = O), 1451 cm− 1 (= C-H), 3241 cm− 1 (O-H), 1558 cm− 1 (C = C), and 3021 − 2961 cm− 1 is for asymmetric and symmetric stretching vibrations of (C-H). For benzoxazine monomer (Bz), The peaks at 1041 and 1218 cm− 1 are correspond to symmetric and asymmetric stretching of (C-O-C), respectively [29,30,31]. The peaks at 1168 and 834 cm− 1 are due to asymmetric and symmetric stretching vibrations of C-N-C of the oxazine ring [29, 31,32,33]. The peaks at 978 and 1512 cm− 1 are attributed to bending tri-substituted benzene ring [33]. The band at 1650 cm− 1 is related to C = O stretching. The band at 1563 cm− 1 is due to asymmetric stretching of C = C benzene ring. The peak at 1351 cm− 1 is related to CH2 wagging of oxazine. The peaks at 2920 and 2957 cm− 1 are assigned to asymmetric stretching CH2. For polybenzoxazine spectrum (PBz), the peaks at 978, 1496 cm− 1 of tri-substituted benzene ring were disappeared and a peak at 1448 cm− 1 was detected, suggesting that, the ring opening took place to afford polybenzoxazine as shown in Fig. 3. Dunker and Ishida [34] were studied the mechanism of benzoxazine polymerization and reported that polymerization was occurred by ring opening of oxazine at the bond of C-O. During this process, tri-substituted benzene ring becomes tetra-substituted. For magnetite nanoparticle (MNP) in Fig. 2, the bands centered at 629, 590 cm− 1 were due to the Fe-O stretching in Fe3O4 which are attributed to tetrahedral and octahedral sites, respectively [35]. The stretching and bending vibrations of –OH group were observed at 3428 and 1634 cm− 1, respectively [36, 37]. Figure 4 shows the FTIR of polybenzoxazine/magnetite composites at different contents (5%, 10%, and 15%) which prepared in different solvents (dioxane and ammonia). The absorption characteristic peaks of both magnetite and benzoxazine were appeared with a slight shifting as a result of interaction between the inorganic material (dispersed phase) and the matrix (polymer chains).
The XRD pattern of magnetite, polybenzoxazine, and polybenzoxazine/magnetite nanocomposite at different concentrations is shown in Fig. 5. For magnetite, the diffraction peaks at 2ϴ=18º, 31º, 36º, 44º, 54º, 58º, and 63º are responded to the (111), (220), (311), (400), (422), (511), and (440) planes of the cubic Fe3O4 lattice, respectively. These results confirm the cubic spinel structure of the magnetite nanoparticles and are in good accord with the XRD patterns of MNPs described in the literature [38, 39]. The average crystallite size of magnetite is 43.4 nm as estimated by the Scherrer equation; D = kλ / βcosθ, where D is particle diameter size, K is Scherrer’s constant (shape factor, 0.94), λ is wavelength of X-ray source (1.541Ǻ), β is the full width at half maxima (FWHM), and θ is the diffraction angle corresponding to the lattice plane. The polybenzoxazine’s XRD results revealed a broad peak at 2ϴ= 20°, supporting its amorphous nature. For nanocomposites, the broad beak of polybenzoxazine and the peak of (311) plane of magnetite at 36º were observed.
The curing behavior of benzoxazine was examined using DSC. The thermograms of benzoxazine monomer (Bz) and polybenzoxazine (PBz) are displayed in Fig. 6. The DSC of the monomer has an exothermic peak at 160 °C, accompanied by an enthalpy of 31.54 Jg− 1 (the exothermic peak associated with benzoxazine ring-opening polymerization). In the DSC of polybenzoxazine (PBz), there is no exothermic peaks was present as a result of completing polymerization, confirming the successful curing process. The lower curing temperature of benzoxazine may be affected by the presence of chalcone moiety (reactive keto- vinyl group of chalcone) and carboxylic group (COOH) in the structure of monomer. These groups can increased the strain around (N–C–O) of oxazine ring and facilitate the ring opening process (C–O cleavage).
The thermal stability of polybenzoxazine and polybenzoxazine/magnetite nanocomposites was tested using TGA as displayed in Fig. 7. The weight residue of nanocomposites was higher than pristine polybenzoxazine as an indication of enhanced the thermal stability due to the addition of magnetite particles, as shown in Table 1. The pristine polymer has a char yield of 12.5% and with the addition of magnetite nanoparticles, the char yield was improved and reached 45, 50.3, and 52% for PBz/MNP-5, PBz/MNP-10, and PBz-/MNP-15, respectively. The weight loss of polybenzoxazine (PBz) was determined at the following temperatures: 27–105 °C (2.6%), 105–280 °C (2.2%), 280–424 °C (33.3%), 424–619 °C (49%), and 619–799.9 °C (0.4%). This means that, more than 50% of the polybenzoxazine (PBz) was stable under the range of 424 °C. For polybenzoxazine, its thermal stability can be explained as follows; a phenolic hydroxyl group is theoretically produced for every oxazine ring that is opened during the polymerization of benzoxazines. Previous work has demonstrated that this hydroxyl group’s hydrogen bonding plays a significant role in the network structure of polybenzoxazines and may be responsible for their mechanical and physical features [40, 41]. Furthermore, the presence of the chalcone in the structure of benzoxazine can increase the crosslinking density and improve the thermal properties of the polymer.
Scanning electron microscopy (SEM) observations of the surface morphology of the polybenzoxazine/magnetite nanocomposite are shown in Fig. 8. It appeared to have a smooth surface morphology (8-a). At different magnifications, the observable particles have polyhedral shapes, which are often related to magnetite particles (8-b,c) with a particle size of 62.3 nm.
The magnetic property of the polybenzoxazine/magnetite nanocomposite was characterized using a vibrating sample magnetometer (VSM). The magnetic property was investigated at room temperature by measuring the magnetization (M) in a magnetic field. To study the effect of medium on the magnetic properties of polybenzoxazine/magnetite nanocomposites, the magnetization was measured for PBz/MNP-15 (which was prepared directly in dioxane), and PBz/MNP-15-Amm (the magnetite was dissolved first in an ammonium solution and then added to the benzoxazine present in dioxane) as shown in Fig. 9, respectively.
It was noticed that, the saturation magnetization (Ms) of polybenzoxazine/magnetite nanocomposite (PBz/MNP-15) was detected at 12 emu/g. The preparation of polybenzoxazine/magnetite nanocomposite with the same magnetite content in a different medium as ammonia solution reduced the value of magnetization to 4.2 emu/g.
Several studies examine the factors that influence the preparation of MNP, such pH [42], type of base used [43], reaction temperature, kind of iron salts, and concentration of ingredients [44]. The method of synthesizing nano-materials represents an important role that will affect the shape, particle size, surface chemistry, particles distribution, and therefore their magnetic properties [45]. Additionally, the method of preparation greatly expresses the degree of structural flaws or impurities in the particle as well as how these flaws are distributed inside the particle, hence dictating its magnetic behavior [46, 47]. There are several methods to synthesize MNP, such as; hydrothermal [48], micro-emulsion technique [49], polyol process [50], sol-gel [51], sonolysis [52], glass crystallization [53], and co-precipitation process [54, 55]. The most suited method among these is co-precipitation because it is economic, simple, and the surface of the nanoparticles produced by this process may be easily modified with other materials [56]. There are several parameters that affect the size and magnetic properties of nanoparticles in the co-precipitation method, such as the type of alkali agent, temperature, time, concentration of reactants, and the way these reactants are added. It is well known that the magnetic properties of MNP are size-dependent, and there are differences in behavior depending on the size of magnetite materials [57]. Generally, the saturation magnetization was found to decrease linearly with decreasing crystallite size [58]. The decreasing of magnetite particle size leads to increase the magnetic properties, but there is an optimum size where the magnetization reduces under this size. It has always been difficult to create magnetite particles with the necessary size and acceptable size distribution without aggregation [50]. A decrease in particle size might exert negative pressure on the lattice, which would cause the lattice volume to increase. The decrease in magnetization as size decreases may be due to surface effect. According to Tajabadi and Khosroshahi [59], magnetite produced at a higher temperature (70 °C) and with less alkaline content has the maximum saturation magnetization. Dimens et al. [56] used ammonium hydroxide as a base to prepare magnetite, the magnetic properties were improved and the magnetization increased with increasing temperatures. Guangze Yang [60] studied the magnetite solubility as a function of temperature and pH (pH 8.7–9.6 was conditioned by adding ammonia). The solubility increased with increasing temperature and reached a maximum at 150 °C. With increasing temperature reaction than 150 °C, the solubility decreased. Wang et al. [61] synthesized water-soluble MNPs with sufficiently high solubility and stability (> one month). It was reported that the MNPs have high magnetization and excellent removal abilities for heavy metals ions (such Pb+2) from waste water. According to Hui et al. [39], in order to create MNPs that are efficient in biochemistry, they must have high dispersion, high stability in air, and high solubility in water (at a pH range of 5–9). To produce pure magnetite, the Fe+2/Fe+3 ratio in the co-precipitation process should remain at the ratio of 1/2. Generally, the common impurities in magnetite reactions are α-Fe2O3 and ɤ-Fe2O3. Although ɤ-Fe2O3 has magnetic properties that are comparable to those of magnetite, but α-Fe2O3 is a nonmagnetic substance that causes undesirable magnetic features in materials. In particular, the impurities of magnetite are a result of Fe+2/Fe+3 ratios that deviate from the target value. Additionally, the oxygen’s presence caused this departure, oxidizing the Fe+2 ions to Fe+3 [62].
In this current study, all experimentation procedures were performed under N2 gas. It was observed that, the magnetization of poly benzoxazine/magnetite nanocomposite decreased in ammonium hydroxide solution but remained higher in dioxane alone. The reduction of magnetic property of polybenzoxazine/magnetite nanocomposite may be due to variety of factors including; (1) increasing solubility of magnetite nanoparticles in ammonia solution, which may degrade some of their magnetic properties by decreasing their size below optimal values. Nedelkoski et al. [63] studied the reduction of magnetization in magnetite at a critical size (less than 15 nm). It may be due to the formation of multiple magnetic domains. (2) Or the presence of this medium may decrease the particle size, but with time the small particles tend to nucleate and aggregate (due to the higher surface energy of the particles) to higher levels of particle size, which reduces the magnetization. Aggregation, i.e., the adhesion of particles, occurs because of Van der Waals attraction. (3) The magnetite surface may be affected by the change in solvent. According to Mariotto et al. [64], the Fe-O ratio on the surface of the magnetite is controlled by the preparation conditions, which have a significant impact on the structure of the mineral. The change in crystallographic structure of magnetite may be reduced the symmetry of cubic system and affected its properties [65]. (4) Also, the change of solvent may affect the Fe+ 2/Fe+ 3 redox reaction and encourage the presence of more impurities or non-magnetic components. Chemical transformations of magnetite may be occurred (as the surface Fe II cations may be reacted with the adsorbed oxygen to form a rim of maghemite). Kodama et al. [66] showed that, a disordered of spins at the magnetite surface reduced the magnetic properties. (5) The (001) surface of magnetite is polar, in accordance with Tasker’s [67] classification of surfaces of ionic or partially ionic materials. So, the presence in ammonia enhanced the solubility of magnetite which may affect its magnetic properties.
Conclusion
A novel benzoxazine monomer was successfully synthesized from hydroxylated chalcone (1-(phenyl)-3(4-hydroxy phenyl)prop-2-en-1-one), paraformaldehyde, and 4-aminobenzoic acid by the solvent method through the Mannich condensation reaction. Thermal curing by ring opening of oxazine was used to create polybenzoxazine. Polybenzoxazine/magnetite nanocomposites were prepared in two media: dioxane and ammonia solution, using different magnetite nanoparticle contents. The medium of composites’ preparation has a significant impact on their magnetic properties. The preparation of polybenzoxazine/magnetite nanocomposite in dioxane leads to a higher value of saturation magnetization (12 emu/g), than those prepared in ammonia solution at the same MNP content (4 emu/g). The resulted benzoxazine monomer has a low curing temperature (160 °C). The addition of magnetite nanoparticles to polybenzoxazine enhanced the thermal stability and increased the char yield.
Data availability
Data will be available on the request.
References
Ishida H, Agag T (2011) Handbook of benzoxazine resins. Elsevier
Rimdusit S, Ishida H (2000) Development of new class of electronic packaging materials based on ternary systems of benzoxazine, epoxy, and phenolic resins. Polym Guildf 41:7941–7949
Spontón M, Lligadas G, Ronda JC et al (2009) Development of a DOPO-containing benzoxazine and its high-performance flame retardant copolybenzoxazines. Polym Degrad Stab 94:1693–1699
Ghosh NN, Kiskan B, Yagci Y (2007) Polybenzoxazines-new high performance thermosetting resins: synthesis and properties. Prog Polym Sci 32:1344–1391
Chirachanchai S, Laobuthee A, Phongtamrug S (2009) Self termination of ring opening reaction of p-substituted phenol-based benzoxazines: an obstructive effect via intramolecular hydrogen bond. J Heterocycl Chem 46:714–721
Low HY, Ishida H (1998) Mechanistic study on the thermal decomposition of polybenzoxazines: Effects of aliphatic amines. J Polym Sci Part B Polym Phys 36:1935–1946
Ishida H, Sanders DP (2000) Regioselectivity and network structure of difunctional alkyl-substituted aromatic amine-based polybenzoxazines. Macromolecules 33:8149–8157
Hemvichian K, Kim HD, Ishida H (2005) Identification of volatile products and determination of thermal degradation mechanisms of polybenzoxazine model oligomers by GC–MS. Polym Degrad Stab 87:213–224
Takeichi T, Kano T, Agag T (2005) Synthesis and thermal cure of high molecular weight polybenzoxazine precursors and the properties of the thermosets. Polym (Guildf) 46:12172–12180
Santhosh Kumar KS, Reghunadhan Nair CP, Sadhana R, Ninan KN (2007) Benzoxazine–bismaleimide blends: curing and thermal properties. Eur Polym J 43:5084–5096
Ishida H, Ohba S (2005) Synthesis and characterization of maleimide and norbornene functionalized benzoxazines. Polym (Guildf) 46:5588–5595
Andreu R, Espinosa MA, Galià M et al (2006) Synthesis of novel benzoxazines containing glycidyl groups: a study of the crosslinking behavior. J Polym Sci Part A Polym Chem 44:1529–1540
Holly FW, Cope AC (1944) Condensation Products of Aldehydes and Ketones with o-Aminobenzyl Alcohol and o-Hydroxybenzylamine. J Am Chem Soc 66:1875–1879
Lin CH, Chien CK, Chen CH, Juang TY (2017) Photo-sensitive benzoxazine II: chalcone-containing benzoxazine and its photo and thermal-cured thermoset. RSC Adv 7:37844–37851
Lin CH, Chen ZJ, Chen CH et al (2017) Synthesis of a bisbenzylideneacetone-containing benzoxazine and its photo- and thermally cured Thermoset. ACS Omega 2:3432–3440
Muthukaruppan A, Arumugam H, Krishnan S et al (2018) A low cure thermo active polymerization of chalcone based benzoxazine and cross linkable olefin blends. J Polym Res 25:163
Davaran S, Rezaei A, Alimohammadi S et al (2014) Synthesis and physicochemical characterization of biodegradable star-shaped poly lactide-co-glycolide-β-cyclodextrin copolymer nanoparticles containing albumin. Adv Nanopart 03:14–22
Meng X, Li H, Chen J et al (2009) Mössbauer study of cobalt ferrite nanocrystals substituted with rare-earth Y3 + ions. J Magn Magn Mater 321:1155–1158
Drummond TG, Hill MG, Barton JK (2003) Electrochemical DNA sensors. Nat Biotechnol 21:1192–1199
Takafuji M, Ide S, Ihara H, Xu Z (2004) Preparation of poly(1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal ions. Chem Mater 16:1977–1983
Mahdavi M, Namvar F, Ahmad M, Bin, Mohamad R (2013) Green biosynthesis and characterization of magnetic iron oxide (fe 3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 18:5954–5964
Socaci C, Rybka M, Magerusan L et al (2013) Magnetite nanoparticles coated with alkyne-containing polyacrylates for click chemistry. J Nanoparticle Res 15:1747
Mustaffa MS, Azis RS, Abdullah NH et al (2019) An investigation of microstructural, magnetic and microwave absorption properties of multi-walled carbon nanotubes/Ni0.5Zn0.5Fe2O4. Sci Rep 9:15523
Tanasa E, Zaharia C, Radu I-C et al (2019) Novel nanocomposites based on functionalized magnetic nanoparticles and polyacrylamide: preparation and complex characterization. Nanomaterials 9
Barrera G, Tiberto P, Allia P et al (2019) Magnetic properties of nanocomposites. Appl Sci 9
Filik H, Avan AA (2019) Magnetic nanostructures for preconcentration, speciation and determination of chromium ions: a review. Talanta 203:168–177
Mandour HSA, Rehab A, Elnahrawy M, Salahuddin N (2022) Synthesis and characterization of chalcone based benzoxazine-magnetite nanocomposites. Chem Pap 76:7565–7574
Kiskan B, Demirel AL, Kamer O, Yagci Y (2008) Synthesis and characterization of nanomagnetite thermosets based on benzoxazines. J Polym Sci Part A Polym Chem 46:6780–6788
Wang J, Fang X, Wu MQ et al (2011) Synthesis, curing kinetics and thermal properties of bisphenol-AP-based benzoxazine. Eur Polym J 47:2158–2168
Liu J, Agag T, Ishida H (2010) Main-chain benzoxazine oligomers: a new approach for resin transfer moldable neat benzoxazines for high performance applications. Polym Guildf 51:5688–5694
Agag T, Jin L, Ishida H (2009) A new synthetic approach for difficult benzoxazines: Preparation and polymerization of 4,4′-diaminodiphenyl sulfone-based benzoxazine monomer. Polym (Guildf) 50:5940–5944
Liu J, Agag T, Ishida H (2010) Main-chain benzoxazine oligomers: a new approach for resin transfer moldable neat benzoxazines for high performance applications. Polym (Guildf) 51:5688–5694
Chozhan CK, Chandramohan A, Alagar M (2019) Cyclohexane and phosphorus based benzoxazine-bismaleimide hybrid polymer matrices: thermal and morphological properties. J Macromol Sci Part A 56:686–696
Dunkers J, Ishida H (1999) Reaction of benzoxazine-based phenolic resins with strong and weak carboxylic acids and phenols as catalysts. J Polym Sci Part A Polym Chem 37:1913–1921
Woo K, Hong J, Ahn JP (2005) Synthesis and surface modification of hydrophobic magnetite to processible magnetite@silica-propylamine. J Magn Magn Mater 293:177–181
Lassoued A, Dkhil B, Gadri A, Ammar S (2017) Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys 7:3007–3015
Aliramaji S, Zamanian A, Sohrabijam Z (2015) Characterization and synthesis of magnetite nanoparticles by innovative sonochemical method. Procedia Mater Sci 11:265–269
Gong J, Lin X (2003) Facilitated electron transfer of hemoglobin embedded in nanosized Fe3O4 matrix based on paraffin impregnated graphite electrode and electrochemical catalysis for trichloroacetic acid. Microchem J 75:51–57
Hui C, Shen C, Yang T et al (2008) Large-Scale Fe 3O 4 nanoparticles soluble in water synthesized by a Facile method. J Phys Chem C 112:11336–11339
Kim H-D, Ishida H (2002) A study on Hydrogen-Bonded Network structure of polybenzoxazines. J Phys Chem A 106:3271–3280
Allen DJ, Ishida H (2007) Polymerization of linear aliphatic diamine-based benzoxazine resins under inert and oxidative environments. Polym (Guildf) 48:6763–6772
Mohammad-Beigi H, Yaghmaei S, Roostaazad R et al (2011) Effect of pH, citrate treatment and silane-coupling agent concentration on the magnetic, structural and surface properties of functionalized silica-coated iron oxide nanocomposite particles. Phys E Low-dimensional Syst Nanostructures 44:618–627
de la Presa P, Luengo Y, Multigner M et al (2012) Study of heating efficiency as a function of concentration, size, and applied field in γ-Fe2O3 nanoparticles. J Phys Chem C 116:25602–25610
Mahadevan S, Gnanaprakash G, Philip J et al (2007) X-ray diffraction-based characterization of magnetite nanoparticles in presence of goethite and correlation with magnetic properties. Phys E Low Dimens Syst Nanostructures 39:20–25
Kouhi M, Vahedi A, Akbarzadeh A et al (2014) Investigation of quadratic electro-optic effects and electro-absorption process in GaN/AlGaN spherical quantum dot. Nanoscale Res Lett 9:131
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144
Grossman HL, Myers WR, Vreeland VJ et al (2004) Detection of bacteria in suspension by using a superconducting quantum interference device. Proc Natl Acad Sci U S A 101:129–134
Xu C, Teja AS (2008) Continuous hydrothermal synthesis of iron oxide and PVA-protected iron oxide nanoparticles. J Supercrit Fluids 44:85–91
Okoli C, Sanchez-Dominguez M, Boutonnet M et al (2012) Comparison and functionalization study of microemulsion-prepared magnetic Iron oxide nanoparticles. Langmuir 28:8479–8485
Hugounenq P, Levy M, Alloyeau D et al (2012) Iron oxide monocrystalline nanoflowers for highly efficient magnetic hyperthermia. J Phys Chem C 116:15702–15712
Xu J, Yang H, Fu W et al (2007) Preparation and magnetic properties of magnetite nanoparticles by sol–gel method. J Magn Magn Mater 309:307–311
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc 7:1–37
Müller R, Hergt R, Dutz S et al (2006) Nanocrystalline iron oxide and Ba ferrite particles in the superparamagnetism-ferromagnetism transition range with ferrofluid applications. J Phys Condens Matter 18
Khalafalla S, Reimers G (1980) Preparation of dilution-stable aqueous magnetic fluids. IEEE Trans Magn 16:178–183
Massart R (1980) Preparation of aqueous ferrofluids without using surfactant behavior as a function of the pH and the counterions CR Hebd. Seances Acad Sci C 291:3
Dimens N (2014) International journal of nano dimension applying a suitable route for preparation Fe 3 O 4 nanoparticles by Ammonia and investigation of their physical and different magnetic properties. 5:297–303
Wallyn J, Anton N, Vandamme TF (2019) Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications a review. Pharmaceutics 11
Morales MP, Andres-Vergés M, Veintemillas-Verdaguer S et al (1999) Structural effects on the magnetic properties of γ-Fe2O3 nanoparticles. J Magn Magn Mater 203:146–148
Tajabadi M, Khosroshahi ME (2012) Effect of alkaline media concentration and modification of temperature on magnetite synthesis method using FeSO4/NH4OH. Int J Chem Eng Appl 3:206–210
Yang G (2017) Investigations of the tube support plate (TSP) clogging phenomenon in PWR steam generators - understanding and prioritization of its formation mechanisms. Universit{é} Pierre et Marie Curie - Paris VI
Wang L, Li J, Jiang Q, Zhao L (2012) Water-soluble Fe 3O 4 nanoparticles with high solubility for removal of heavy-metal ions from waste water. Dalt Trans 41:4544–4551
Maity D, Agrawal DC (2007) Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J Magn Magn Mater 308:46–55
Nedelkoski Z, Kepaptsoglou D, Lari L et al (2017) Origin of reduced magnetization and domain formation in small magnetite nanoparticles. Sci Rep 7:45997
Mariotto G, Murphy S, Shvets IV (2002) Charge ordering on the surface of Fe3O4 (001). Phys Rev B 66:245426
Verwey EJ, Haayman PW, Romeijn FC (1947) Physical properties and cation arrangement of oxides with spinel structures II. Electronic conductivity. J Chem Phys 15:181–187
Kodama RH, Berkowitz AE, McNiff EJ Jr, Foner S (1996) Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett 77:394–397
Tasker PW (1979) The stability of ionic crystal surfaces. J Phys C Solid State Phys 12:4977–4984
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mandour, H.S.A., Rehab, A., Elnahrawy, M. et al. The effect of preparation conditions of chalcone based benzoxazine/magnetite nanocomposites on magnetization properties. J Polym Res 30, 144 (2023). https://doi.org/10.1007/s10965-023-03515-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03515-x