Abstract
Here, we proposed an approach to develop magnetic chalcone based benzoxazine using different contents of magnetite nanoparticles. A chalcone containing benzoxazine was prepared from 3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one), stearyl amine and paraformaldehyde through Mannich condensation reaction in a cosolvent of ethanol/toluene (1/1)(v/v). The chemical structure of the prepared benzoxazine monomer was confirmed by FTIR and 1H NMR. Both monomer and monomer mixed with different contents of magnetite were exposed to UV irradiation to induce dimerization via [2p + 2p] cycloaddition followed by thermal curing of oxazine moiety. The crystal structure of magnetite nanoparticles was studied by X-ray diffraction (XRD) analysis. Scanning electron microscope (SEM) was used to examine the surface morphology of the resulted materials. The average size of magnetite nanoparticles was determined by transmission electron microscope (TEM) to be 30–40 nm. The magnetization properties of these materials were measured by vibrating sample magnetometer (VSM). The thermal properties of thermosets were evaluated and compared with nanocomposites using TGA and DSC. The thermosets exhibited good thermal stability and improved with increasing the magnetite contents in the feed.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Structured materials must be easy to manufacture, exhibit a long shelf life, and preserve their mechanical properties over a wide range of temperatures (Soto et al. 2016). Phenolic thermosetting resins have some of these requirements due to their good mechanical strength, thermal stability, structural integrity, chemical resistance, and low cost owing to the inexpensive raw materials and fabricating processes (Takeichi et al. 2008). However, some limitations that reduce the applications of phenolic-based materials as the use of harsh catalyst for polymerization, evolution of volatiles during curing process, and the brittleness of the crude materials (Li et al. 2014; Soto et al. 2016). Polybenzoxazine (PBz) are a versatile group of phenolic polymers that have excellent heat resistance including, low dielectric properties, flame-retardant, superior membrane properties at high temperature, low surface energy, low shrinkage, and a high char yield. In addition, no strong acid catalysts are required for curing, no by-products are released during curing and low water absorption (Agag et al. 2009; Cao et al. 2008; Ghosh et al. 2007; Wang and Ishida 1999). The variety of properties help the polybenzoxazine to meet the performance requirements of some demanding applications. These include areas as diverse as automotive, aerospace, and electronics industry. The aromatic polymer backbone and extensive inter–intra hydrogen bonds offer superior thermal and mechanical properties. The wide range of mechanical and thermal properties renders polybenzoxazine to be tailored to various applications due to the extremely flexible molecular designs. Polybenzoxazine can be easily prepared from an inexpensive raw compounds like amines, phenols, and paraformaldehyde without using catalyst (Ke et al. 2012; Liu et al. 2010; Jin et al. 2011; Zhang et al. 2018). However, benzoxazine-based polymers offer several disadvantages including their high brittleness, high curing temperatures, and the difficulties in preparing films. The use of phenol to synthesize polybenzoxazine may create significant threats to environment as well as human health.
To enhance the properties of (PBz), studies were done on the curing mechanism (Kawaguchi et al. 2014; Kudoh et al. 2010; Oie et al. 2012; Sini and Endo 2016) by incorporation of a multifunctional linkage (Shukla and Lochab 2016; Wang et al. 2014; Zhang et al. 2015), photo-sensitive moieties such as methacryloyl (Jin et al. 2011; Koz et al. 2011), coumarins (Lin et al. 2016; Matsumura et al. 2008; Mohamed et al.2015), chalcone moieties with polyimides (Song et al. 2003; Tie et al. 2012), methacrylate homopolymer (Balaji and Nanjundan 2001; Balamurugan et al. 2012; Rehab and Salahuddin 1999), chalcones containing epoxy (Choi, et al. 2001), fluorinated chalcone containing poly(arylene ether) and poly(arylene ether sulfone) (Tie et al. 2012). The incorporation of photosensitive moieties such as coumarins, chalcones and methacryloylchloride into polybenzoxazine appeared to be an alternative strategy to afford high performance benzoxazine thermosets. It was reported that, upon irradiation of coumarin at wavelength higher than 310 nm, it undergoes photodimerization through the cyclobutene formation. A chalcone containing benzoxazine-thermosets that produced by photo curing followed by thermal curing exhibited a glass transition temperature higher than thermosets cured thermally. Photo-cured chalcone moiety afford tetrafunctional benzoxazine leading to higher cross-linking density and synthesis of chalcone based benzoxazine was reported earlier (Feng et al. 2022; Hariharan et al. 2020; Lin et al. 2017; Muthukaruppan et al. 2018). For applications in harsh conditions, several studies were done to improve the thermal properties of polybenzoxazine by using bifunctional monomers, blending with high performance monomers, incorporation nanomaterials or by preparing specially designed novel monomers.
Magnetite nanoparticles (MNPs) have wide range of applications including environmental remediation, data storage, biomedicine, catalysis and magnetic fluids, magnetic resonance imaging (MRI), drug delivery systems, medical diagnostics, cancer therapy, microwave devices, and magneto-optic devices (Davaran et al. 2013; El Ghandoor et al. 2012; Ghasemali et al. 2013; Meng et al. 2009) due to their multifunctional properties such as high surface area, superparamagnetism and low toxicity (Ahmadi et al. 2014; Biehl et al. 2018; Gu et al. 2006). However, the MNPs have high chemical activity, and are easily oxidized in air, resulting in the loss of magnetism and dispersibility.
High curing temperature as well as low toughness of the thermosets are considered as limitations for large scale applications. The lowering of curing temperature of polybenzoxazine still remains a challenge in addressing their exploration in low-temperature processing applications. Monisha et al (2018) proposed an approach to develop a composite based on smartly-capped iron nanoparticles and agro-waste phenolic-sourced cardanol benzoxazine monomer with lowering the temperature of curing (Monisha et al. 2018).
In this work, a chalcone was prepared via aldol condensation reaction. Then, benzoxazine monomer was prepared using chalcone, 1-(phenyl)-3(4 hydroxy phenyl)prop-2-en-1-one), stearyl amine and paraformaldehyde through Mannich condensation reaction. After that, chalcone based benzoxazine was prepared by photo and thermal curing of oxazine moiety to produce high performance thermosets. The presence of double bonds in the chalcone moiety can be formed a cyclobutene ring during the exposure of UV irradiation and affecting the thermal properties of the resulting resins. The effect of magnetite on the thermal properties of the thermosets are studied.
Experimental
Materials
4-hydroxy benzaldehyde (LOBA-Chemie), acetophenone (ADWIC-Egypt), boron trifluoride etherate (BF3.Et2O) (Tokyo Chemical Industry Co., Ltd. (JP)) paraformaldehyde (Merck, Germany), stearylamine, ferric chloride hexahydrate (FeCl3.6H2O), ferrous sulfate heptahydrate (FeSO4.7H2O), anhydrous sodium sulfate (anhyd.Na2SO4) (Tokyo Chemical Industry Co., Ltd. (JP)), ether (ADWIC-Egypt), absolute ethanol (Bio.Chem), toluene (NaTco.laboratory chemicals reagent), 1,4 dioxane (Sd Fine Chem limited-India), oleic acid (Qualikems-India) were used without further purifications.
Preparation of hydroxylated chalcone
According to the previously reported method (Narender and Papi Reddy 2007) with slight modification as shown in Scheme 1, BF3-Et2O (3.6 mL, 30 mmol) was gradually added at room temperature to a stirred solution of acetophenone (1) (7.2 g, 7 mL, 60 mmol) and 4-hydroxybenzaldehyde (2) (7.32 g, 30 mmol) in 50 mL dioxane. The solution was stirred for 15 min at room temperature and the reaction mixture was diluted with ether (250 mL). Then, the solution was washed with water (150 mL) to discharge the color and the BF3–Et2O complex. The obtained ether layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The crude product (3) was purified by column chromatography to afford the desired chalcone (11.96 g, 89%). 1H NMR (400 MHz, CDCl3) δ 5.71 (s, 1H), 6.86 (d, 2H), 7.36 (d,1H), 7.46–7.58 (m, 5H), 7.73 (d, 1H), 7.96–800 (m, 2H) ppm.
Preparation of benzoxazine monomer (Bz monomer)
Benzoxazine monomer (4) was synthesized as shown in scheme 1; paraformaldehyde (3 g, 100 mmol) and stearyl amine (13.5 g, 50 mmol) were mixed in a 250 ml three-necked round-bottom flask under vigorous stirring in the presence of cosolvent (60 mL ethanol–60 mL toluene) at 5 °C for 1 h. After that, chalcone 3 (11.2 g, 50 mmol) was added to the mixture and stirring was continued. The temperature was then gradually raised to 110 °C and the reaction was refluxed for 48 h. Then, the reaction mixture was cooled, filtered, washed by cold ethanol, and finally dried under vacuum to afford the desired Bz monomer (4). The molecular structure of Bz monomer was confirmed with IR, 1H NMR spectroscopy. 1H NMR spectrum of benzoxazine monomer (Bz monomer) (4), (400 MHz, CDCl3) reveals δ 0.87 (t, 3H), 1.25 (s, 32H), 2.73 (t,2H), 4.02 (s, 2H), 4.92 (s, 2H), 6.79 (d, 1H), 7.27 (s, 1H), 7.37 (d, 1H), 7.45 (dd, 1H), 7.49 (d, 2H), 7.56–7.59 (m, 1H), 7.72 (d, 1H), 7.99–8.02 (m, 2H) ppm.
Curing of benzoxazine monomer
Polybenzoxazines were obtained by the thermal ring-opening polymerization of benzoxazine monomers via a cationic mechanism (Ran et al. 2012). In the thermal curing of benzoxazines, the monomer was exposed to heating in an oven at 180 °C for 1 h to obtain (PBz-Th180), a further curing at 200 °C for 1 h leads to (PBz-Th200) and finally the last curing at 230 °C for 2 h leads to (PBz-Th230). For photo-curing of benzoxazine, benzoxazine monomer dissolved in 1,4 dioxane was irradiated by UV (100 W, 365 nm) for 45 min followed by evaporation the solvent at 70–80 °C for 24 h, cured at 180 °C for 1 h to obtain (PBz-UVTh180), a further curing at 200 °C for 1 h afforded (PBz-UVTh200). The last curing at 230 °C for 2 h afforded (PBz-UVTh230). Photo-curing deals with the chalcone moiety only, followed by thermal curing which deals with the oxazine moiety.
Preparation of magnetite nanoparticles (MNP)
The magnetite nanoparticles were synthesized according to the previously reported method (Matsumura et al. 2008). In 500 ml round two neck flask, ferric chloride hexahydrate FeCl3.6H2O (5 g, 25 mmol) and ferrous sulfate heptahydrate FeSO4.7H2O (2.3 g, 12.5 mmol) were dissolved in 250 ml distilled water under nitrogen and stirred for 30 min at 50 °C. Then, 100 ml of sodium hydroxide (1.5 M) was added drop wise and stirred. After 24 h, the precipitate was separated and washed with distilled water and absolute ethanol, dried at 50 °C under vacuum to afford 6.1 g MNP.
Synthesis of benzoxazine-magnetite nanoparticles composite (PBz-MNP)
0.425 g of benzoxazine monomer (Bz) (4) was dissolved in 15 ml of 1,4 dioxane, 0.075 g of magnetite nanoparticle (MNP) was added to the benzoxazine solution followed by addition of 2 ml of oleic acid and the mixture was sonicated for 15 min at 40 °C. After that, the solvent was evaporated at 70 °C and the resulted material was cured thermally at 230 °C to obtain PBz-Th230/15-MNP. The same procedure was done using (0.475 g, 0.45 g) of Bz-4 and (0.025 g, 0.05 g) of magnetite to afford PBz-Th230/5-MNP and PBz-Th230/10-MNP, respectively.
Characterization
FTIR spectra were measured using a PerkinElmer 1420 spectrometer using KBr Disc technique with a frequency range from 4000 to 400 cm−1. XRD data were measured by an auto-diffractometer which consist of a Philips XRG 3100 X (λ = 1.5418 Å) X-ray source, connected to a Phillips APD 3520 type PW1710 diffractometer controller. Proton nuclear magnetic resonance (1H NMR) spectra were obtained by means of a Bruker at 400 MHz using CDCl3 as a solvent. Differential scanning calorimetry (DSC) scans were recorded using a Shimadzu-60-DSC in a nitrogen atmosphere at a heating rate of 10 °C/min with gas flow 20 ml/min. Thermal gravimetric analysis (TGA) was determined using a Shimadzu TGA–50 H thermal analyzer system in the temperature range of 30–800 °C under nitrogen atmosphere with flow rate of 40 ml/min. The surface morphology of MNP and nanocomposites was examined, using a scanning electron microscope (SEM) (JSM-6400 JEOL). Transmission Electron Microscopy (TEM) (JEOL JEM100–IDO-SX (JEOL Ltd., Japan)) was used with an accelerating voltage of 20 kV. Magnetic properties of samples were measured at room temperature using a magnetometer (VSM) with a magnetic field up to 10 kOe. The UV irradiation was done using UV lamp (100 W, 365 nm).
Results and discussion
1H NMR spectrum of benzoxazine monomer 4 is shown in Fig. 1, the methyl group, (-CH3) was detected at 0.87 ppm, methylene units of stearyl group were appeared at 1.25, 2.73 ppm. The characteristic peaks of benzoxazine ring were appeared at 4.02 and 4.92 ppm. These characteristic peaks are attributed to the methylene groups (Ar–CH2–N) and (O–CH2–N) of oxazine ring, respectively. It was observed that, no signal of phenolic OH, suggesting that the Mannich condensation was successfully occurred. The aromatic protons were identified in the range between 6.79 and 8.02 ppm.
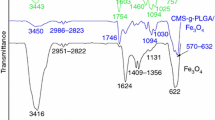
Figure 2 shows FTIR spectra of chalcone, Bz monomer, PBz-Th230, PBz-UVTh230, PBz-Th230/5-MNP, PBz-Th230/10-MNP, and PBz-Th230/15-MNP nanocomposites. The spectrum of chalcone reveals the band at 1650 cm−1 (C=O in conjugation with C=C), bands at 1562,1595 cm−1 (C═C which conjugated to the ketone group), band at 3240 cm−1 (O–H). Asymmetric and symmetric stretching vibrations of the aromatic C–H bonds were observed near 3020, 2970 cm−1. The in-plane deformation of the =C–H bond is appeared at 1450 cm−1. For Bz monomer, the characteristic out-of-plane absorption modes of benzene ring with an attached oxazine ring is located at 905, 935 cm−1. The band at 1500 cm−1 is characteristic mode of tri-substituted benzene in the benzoxazine structure. The existence of a benzoxazine ring in the monomer was specified by the band centered at 1237 cm−1 which is due to the C–O–C asymmetric stretching modes (Agag and Takeichi 2003). The bands at 2852, 2921 cm−1 were due to C–H bands (stearyl amine). The band at 1169 cm−1 was related to the C–N–C asymmetric stretching mode. The band at 1017 cm−1 was assigned to the symmetric stretching mode of C–O–C of the oxazine ring. The peak of CH2 wagging was observed at 1318, 1344 cm−1 (Kiskan et al. 2011). For polybenzoxazine spectrum, the peak at 935 cm−1 was disappeared in the thermally cured polybenzoxazine (PBz-Th230) and a new peak centered at 1470 cm−1, representing tetra-substituted benzene ring mode.
For magnetite MNP, the bands assigned at 590, 629 cm−1 were related to the Fe–O stretching vibration in Fe3O4 which are attributed to octahedral and tetrahedral, respectively. The stretching and deformation vibration of –OH was observed at 3428 and 1634 cm−1, respectively, which is assigned to –OH adsorbed by Fe3O4 nanoparticles. For PBz-Th230, PBz-Th230/5-MNP, PBz-Th230/10-MNP, and PBz-Th230/15-MNP nanocomposites, the characteristic peaks of both polybenzoxazine and magnetite were observed with slight shifts due to the interaction between the polymer and the inorganic material.
The DSC thermograms of Bz monomer, PBz-Th230, PBz-UV-Th230 are shown in Fig. 3. The polymerization of benzoxazine monomer was at the range of 170 °C which is related to the ring opening of oxazine ring. Benzoxazines typically show a symmetric exotherm in the range of 180–250 °C (Jayamohan Das et al. 2013; Hamerton et al. 2013). It was observed that the exothermic peak after polymerization process was disappeared due to the complete process of polymerization.
The XRD pattern of the MNP, PBz-Th230/5-MNP, PBz-Th230/10-MNP and PBz-Th230/15-MNP nanocomposites is shown in Fig. 4. The XRD spectrum of magnetite nanoparticles exhibited peaks at 2ɵ = 18º, 31º, 36º, 44º, 54º, 58º, and 63º that can be indexed to (111), (220), (311), (400), (422), (511), and (440) which agree with the literature (Gong and Lin 2003; Liao and Chen 2001). For PBz-Th230 a broad peak was observed at 2ɵ = 20 º confirming the amorphous structure. For nanocomposites, the peaks corresponding to magnetite were observed to some extent especially at the low concentration of magnetite (5%). With increasing the amount of magnetite in the composites the peaks of magnetite seem more obviously.
VSM of (a) PBz-Th-230/15-MNP and PBz-Th-230/10 MNP nanocomposites. Scanning electron microscopy (SEM) was used to investigate the morphology of the PBz-Th-230/15-MNP. SEM micrographs of these nanocomposites reveal that the MNP moieties remained dispersed within the polybenzoxazine matrix. The degree of dispersion of the nanofiller plays an important role in influencing the properties of the resulting nanocomposites. With high magnification, a considerable number of the magnetite nanoparticles were appeared as a uniform cluster
The magnetic properties of PBz-Th230/15-MNP and PBz-Th230/10-MNP nanocomposites were investigated by measuring the magnetization M with magnetic field at room temperature using vibrating sample magnetometer (VSM). It was observed that the insertion of the MNP into polybenzoxazine did not prevent the magnetic property of these nanoparticles. The saturation magnetization (Ms) of PBz-Th230/15-MNP and PBz-Th230/10-MNP was 10.14 and 7.3 emu/g, respectively. The small value of magnetization is assumed to nonmagnetic polybenzoxazine. The interesting feature was that the pure MNP remains almost unaffected in the composites. It is well known that aggregation of magnetic particles displays a demagnetizing effect due to random dipole–dipole interactions between particles resulting in antiferromagnetic coupling (Gross et al. 2003; Nakata et al. 2008). Hence, the enhancement of magnetic properties of the benzoxazine/MNP composites is attributed to the better dispersion behavior of the MNP (Fig. 5).
TEM was performed to analyze the internal morphology of polybenzoxazine/magnetite nanocomposites (Fig. 6b). The micrograph shows dark spherical particles ranged from 30 to 40 nm, which are uniformly dispersed in the composite. These dark portions are attributed to magnetite nanoparticles. Moreover, there is no aggregation of MNP moieties even after complete curing, showing uniform distribution of MNP in poly benzoxazine chains.
The thermal stability of PBz-Th230, PBz-Th230/5-MNP, PBz-Th230/10-MNP, and PBz-Th230/15-MNP was studied by thermogravimetric analysis (TGA) under nitrogen atmosphere as shown in Fig. 7. The thermal degradation of PBz-Th230 has three stages: degradation of the chain ends, evaporation of the amine, and the simultaneous breakage and degradation of the Mannich base (Low and Ishida 1998). The addition of magnetite to the PBz-Th230 improved the thermal stability compared with pristine polybenzoxazine. The char yield was increased from 20.2% for the polybezoxazine to 27, 31.8, and 40.2% for PBz-Th230/5-MNP, PBz-Th230/10-MNP, and PBz-Th230/15-MNP, respectively. The important stages of degradation process of benzoxazine polymer (PBz) and benzoxazine/magnetite composites at different concentrations (5–15%) with the weight loss and char yield were mentioned in Table 1.
Conclusion
A magnetic chalcone contains benzoxazine was successfully prepared. The benzoxazine monomer was synthesized from 3-(4-hydroxyphenyl)-1-phenylprop-2-en-1-one, stearyl amine and paraformaldehyde through Mannich condensation reaction in a cosolvent of ethanol/toluene (1/1). The magnetic poly benzoxazine nanocomposites were prepared using different contents of magnetite. The incorporation of chalcone into benzoxazine was considered a good way to afford high-performance benzoxazine thermosets. Two curing procedures were applied for benzoxazine, thermal curing of the chalcone with oxazine moieties and photo curing of the chalcone moiety, followed by thermal curing of the oxazine moiety. An exothermic peak was observed near to 170 ºC from DSC thermogram which is resulted from the ring opening of curing process. The chalcone moiety was photo-cured to form tetrafunctional benzoxazine which can increase the cross-linking of the thermoset and enhanced its thermal properties. The addition of magnetite into benzoxazine improved the thermal stability and increased the char yield from 20.2% for the polybenzoxazine to 40% for MNP/benzoxazine composites. TEM image revealed that, there is no aggregation of MNP moieties even after complete curing, showing uniform distribution of MNP in the benzoxazine chains with a size range (30–40 nm). It was observed that the insertion of the MNP into poly benzoxazine did not prevent the magnetic property of these nanoparticles.
References
Agag T, Takeichi T (2003) Synthesis and characterization of novel benzoxazine monomers containing allyl groups and their high performance thermosets. Macromolecules 36(16):6010–6017
Agag T, Jin L, Ishida H (2009) A new synthetic approach for difficult benzoxazines: preparation and polymerization of 4,4′-diaminodiphenyl sulfone-based benzoxazine monomer. Polymer (guildf) 50(25):5940–5944
Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K (2014) An electrochemical immunosensor for digoxin using core-shell gold coated magnetic nanoparticles as labels. Mol Biol Rep 41(3):1659–1668
Balaji R, Nanjundan S (2001) Synthesis and characterization of photocrosslinkable functional polymer having pendant chalcone moiety. React Funct Polym 49(1):77–86
Balamurugan S, Nithyanandan S, Selvarasu C, Yeap GY, Kannan P (2012) Photophysical and photochemical investigations on triazole ring linked chalcone containing polymethacrylates. Polymer (guildf) 53(19):4104–4111
Biehl P, von der Lühe M, Dutz S, Schacher FH (2018) Synthesis, characterization, and applications of magnetic nanoparticles featuring polyzwitterionic coatings. Polymers (Basel) 10(1)
Cao GP, Chen WJ, Liu XB (2008) Synthesis and thermal properties of the thermosetting resin based on cyano functionalized benzoxazine. Polym Degrad Stab 93(3):739–744
Choi DH, Oh SJ, Cha HB, Lee JY (2001) Photochemically bifunctional epoxy compound containing a chalcone moiety. Eur Polym J 37(10):1951–1959
Davaran S, Akbarzadeh A, Nejati-Koshki K, Alimohammadi S, Farajpour Ghamari M, Mahmoudi Soghrati M (2013) In vitro studies of NIPAAM-MAA-VP copolymer-coated magnetic nanoparticles for controlled anticancer drug release*. J Encapsulation Adsorpt Sci 03(04):108–115
El Ghandoor H, Zidan HM, Khalil MMH, Ismail MIM (2012) Synthesis and some physical properties of magnetite (Fe 3O 4) nanoparticles. Int J Electrochem Sci 7(6):5734–5745
Feng Z, Zeng M, Tan D, Lu X, Shen Y, Xu Q (2022) Two photosensitive chalcone-based benzoxazine monomers and their high-performance polymers from renewable sources. J Mater Sci 57(7):4895–4913
Ghasemali S, Nejati-Koshki K, Akbarzadeh A, Tafsiri E, Zarghami N, Rahmati-Yamchi M (2013) Inhibitory effects of ß-cyclodextrin-helenalin complexes on H-TERT gene expression in the T47D breast cancer cell line—results of real time quantitative PCR. Asian Pacific J Cancer Prev 14(11):6949–6953
Ghosh NN, Kiskan B, Yagci Y (2007) Polybenzoxazines-new high performance thermosetting resins: synthesis and properties. Prog Polym Sci 32(11):1344–1391
Gong J, Lin X (2003) Facilitated electron transfer of hemoglobin embedded in nanosized Fe3O4 matrix based on paraffin impregnated graphite electrode and electrochemical catalysis for trichloroacetic acid. Microchem J 75(1):51–57
Gross AF, Diehl MR, Beverly KC, Richman EK, Tolbert SH (2003) Controlling magnetic coupling between cobalt nanoparticles through nanoscale confinement in hexagonal mesoporous silica. J Phys Chem B 107(23):5475–5482
Gu H, Xu K, Xu C, Xu B (2006) Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem Commun 9:941–949
Hamerton I, Ennis K, Howlin BJ, McNamara LT (2013) Effects of thermal history on the polymerisation mechanism and network development in aromatic polybenzoxazines. React Funct Polym 73(12):1612–1624
Hariharan A, Prabunathan P, Subramanian SS, Kumaravel M, Alagar M (2020) Blends of chalcone benzoxazine and bio-benzoxazines coated cotton fabrics for oil–water separation and bio-silica reinforced nanocomposites for low-k applications. J Polym Environ 28(2):598–613
Jayamohan Das DL, Rajeev R, Rajeev RS, Santhosh Kumar KS (2013) Synthesis, characterization, curing and thermal decomposition kinetics of bisphenol-a based polybenzoxazine. Int J Sci Technol Res 2(10):146–155
Jin L, Agag T, Yagci Y, Ishida H (2011) Methacryloyl-functional benzoxazine: photopolymerization and thermally activated polymerization. Macromolecules 44(4):767–772
Kawaguchi AW, Sudo A, Endo T (2014) Functional 1,3-benzoxazine bearing 4-pyridyl group: synthesis and thermally induced polymerization behavior. J Polym Sci Part A Polym Chem 52(3):410–416
Ke L, Hu D, Lu Y, Feng S, Xie Y, Xu W (2012) Copolymerization of maleimide-based benzoxazine with styrene and the curing kinetics of the resultant copolymer. Polym Degrad Stab 97(2):132–138
Kiskan B, Ghosh NN, Yagci Y (2011) Polybenzoxazine-based composites as high-performance materials. Polym Int 60(2):167–177
Koz B, Kiskan B, Yagci Y (2011) A novel benzoxazine monomer with methacrylate functionality and its thermally curable (co)polymers. Polym Bull 66(2):165–174
Kudoh R, Sudo A, Endo T (2010) A highly reactive benzoxazine monomer, 1-(2-Hydroxyethyl)-1,3-Benzoxazine: activation of benzoxazine by neighboring group participation of hydroxyl group. Macromolecules 43(3):1185–1187
Li S, Wang H, Tao M (2014) Synthesis and characterization of a new reactive hyperbranched polyphosphate ester, and its modification on benzoxazine-bisoxazoline resins. Des Monomers Polym 17(7):693–699
Liao MH, Chen DH (2001) Immobilization of yeast alcohol dehydrogenase on magnetic nanoparticles for improving its stability. Biotechnol Lett 23(20):1723–1727
Lin RC, Mohamed MG, Hsu KC, Wu JY, Jheng YR, Kuo SW (2016) Multivalent photo-crosslinkable coumarin-containing polybenzoxazines exhibiting enhanced thermal and hydrophobic surface properties. RSC Adv 6(13):10683–10696
Lin CH, Chien CK, Chen CH, Juang TY (2017) Photo-sensitive benzoxazine II: chalcone-containing benzoxazine and its photo and thermal-cured thermoset. RSC Adv 7(60):37844–37851
Liu J, Agag T, Ishida H (2010) Main-chain benzoxazine oligomers: a new approach for resin transfer moldable neat benzoxazines for high performance applications. Polymer (guildf) 51(24):5688–5694
Low HY, Ishida H (1998) Mechanistic study on the thermal decomposition of polybenzoxazines: effects of aliphatic amines. J Polym Sci Part B Polym Phys 36(11):1935–1946
Matsumura S, Hlil AR, Lepiller C, Gaudet J, Guay D, Shi Z (2008) Stability and utility of pyridyl disulfide functionality in RAFT and conventional radical polymerizations. J Polym Sci Part A Polym Chem 46:7207–7224
Meng X, Li H, Chen J, Mei L, Wang K, Li X (2009) Mössbauer study of cobalt ferrite nanocrystals substituted with rare-earth Y3+ ions. J Magn Magn Mater 321(9):1155–1158
Mohamed MG, Hsu KC, Kuo SW (2015) Bifunctional polybenzoxazine nanocomposites containing photo-crosslinkable coumarin units and pyrene units capable of dispersing single-walled carbon nanotubes. Polym Chem 6(13):2423–2433
Monisha M, Yadav N, Srivastava SB, Singh SP, Lochab B (2018) Sustainable one-step strategy towards low temperature curable superparamagnetic composite based on smartly designed iron nanoparticles and cardanol benzoxazine. J Mater Chem A 6(6):2555–2567
Muthukaruppan A, Arumugam H, Krishnan S, Kannan K, Chavali M (2018) A low cure thermo active polymerization of chalcone based benzoxazine and cross linkable olefin blends. J Polym Res 25(8):1556–1559
Nakata K, Hu Y, Uzun O, Bakr O, Stellacci F (2008) Chains of superparamagnetic nanoparticles. Adv Mater 20(22):4294–4299
Narender T, Papi Reddy K (2007) A simple and highly efficient method for the synthesis of chalcones by using borontrifluoride-etherate. Tetrahedron Lett 48(18):3177–3180
Oie H, Mori A, Sudo A, Endo T (2012) Synthesis of networked polymer based on ring-opening addition reaction of 1,3-benzoxazine with resorcinol. J Polym Sci Part A Polym Chem 50(22):4756–4761
Ran QC, Zhang DX, Zhu RQ, Gu Y (2012) The structural transformation during polymerization of benzoxazine/FeCl3 and the effect on the thermal stability. Polymer (guildf) 53(19):4119–4127
Rehab A, Salahuddin N (1999) Photocrosslinked polymers based on pendant extended chalcone as photoreactive moieties. Polymer (guildf) 40(9):2197–2207
Shukla S, Lochab B (2016) Role of higher aromatic content in modulating properties of cardanol based benzoxazines. Polymer (guildf) 99:684–694
Sini NK, Endo T (2016) Toward elucidating the role of number of oxazine rings and intermediates in the benzoxazine backbone on their thermal characteristics. Macromolecules 49(22):8466–8478
Song DM, Jung KH, Moon JH, Shin DM (2003) Photochemistry of chalcone and the application of chalcone-derivatives in photo-alignment layer of liquid crystal display. Opt Mater (amst) 21(1–3):667–671
Soto M, Hiller M, Oschkinat H, Koschek K (2016) Multifunctional benzoxazines feature low polymerization temperature and diverse polymer structures. Polymers (Basel) 8(8):1–14
Takeichi T, Kawauchi T, Agag T (2008) High performance polybenzoxazines as a novel type of phenolic resin. Polym J 40(12):1121–1131
Tie W, Zhong Z, Li L, Zhang A, Shen F, Lee MH (2012) Synthesis and characterization of novel photosensitive polysulfones with photocrosslinkable side pendants. Eur Polym J 48(12):2070–2075
Wang YX, Ishida H (1999) Cationic ring-opening polymerization of benzoxazines. Polymer (guildf) 40(16):4563–4570
Wang H, Wang J, He X, Feng T, Ramdani N, Luan M (2014) Synthesis of novel furan-containing tetrafunctional fluorene-based benzoxazine monomer and its high performance thermoset. RSC Adv 4(110):64798–64801
Zhang T, Wang J, Feng T, Wang H, Ramdani N, Derradji M (2015) A novel high performance oxazine derivative: Design of tetrafunctional monomer, step-wise ring-opening polymerization, improved thermal property and broadened processing window. RSC Adv 5(42):33623–33631
Zhang W, Zhan Y, Gao X, Li R, Zhu W, Xu H (2018) Effect of oxygen functionalities of graphene oxide on polymerization and thermal properties of reactive benzoxazine nanocomposites. Macromol Res 26(1):77–84
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mandour, H.S.A., Rehab, A., Elnahrawy, M. et al. Synthesis and characterization of chalcone based benzoxazine-magnetite nanocomposites. Chem. Pap. 76, 7565–7574 (2022). https://doi.org/10.1007/s11696-022-02400-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02400-z