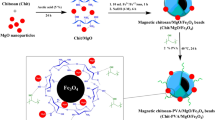
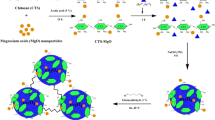
Abstract
In this research work, a hybrid organic–inorganic magnetic nanocomposite of magnetic chitosan-ethylene glycol diglycidyl ether/magnesium oxide (CS-EGDE/MgO/Fe3O4) was prepared as a bioadsorbent material for dye wastewater treatment (reactive blue 19, RB 19). A response surface methodology (RSM) was adopted for the design of 29 experiments to investigate the effect of four factors [A: CS-EGDE/MgO/Fe3O4 dose (0.02–0.1 g), B: pH (4–10), C: temperature (30–60 °C), and time D: (20–80 min)] related to the adsorption of RB 19. The Freundlich adsorption isotherm and the pseudo-second-order kinetics model favourably describe the adsorption of RB 19 by CS-EGDE/MgO/Fe3O4. The CS-EGDE/MgO/Fe3O4 nanocomposite showed a maximum absorption capacity of 133.8 mg/g with the RB 19 dye at 45 °C. The adsorption mechanism for CS-EGDE/MgO/Fe3O4-RB 19 dye system involves electrostatic attraction, H- bonding, n-π interaction, and Yoshida H-bonding. This study supports that the nanocomposite system is an efficient bioadsorbent, which can be deployed for effective decontamination of water that contains acidic dye species.







Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdulhameed AS, Firdaus Hum NNM, Rangabhashiyam S, Jawad AH, Wilson LD, Yaseen ZM, Al-Kahtani AA, ALOthman ZA (2021) Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass based activated carbon using K2CO3 activator. J Environ Chem Eng 9:105530–105542
Mubarak NSA, Jawad AH, Nawawi WI (2017) Equilibrium, kinetic and thermodynamic studies of Reactive Red 120 dye adsorption by chitosan beads from aqueous solution. Energ Ecol Environ 2(1):85–93
Yang W, Zhou M, Mai L, Ou H, Oturan N, Oturan MA, Zeng EY (2021) Generation of hydroxyl radicals by metal-free bifunctional electrocatalysts for enhanced organics removal. Sci Total Environ 791:148107
Kumar S, Krishnakumar B, Sobral AJFN, Koh J (2019) Bio-based (chitosan/PVA/ZnO) nanocomposites film: Thermally stable and photoluminescence material for removal of organic dye. Carbohydr Polym 205:559–564
Nemiwal M, Zhang TC, Kumar D (2021) Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci Total Environ 767:144896
Yurtsever A, Basaran E, Ucar D (2020) Process optimization and filtration performance of an anaerobic dynamic membrane bioreactor treating textile wastewaters. J Environ Manage 273:111114
Azari A, Nabizadeh R, Nasseri S, Mahvi AH, Mesdaghinia AR (2020) Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 250:126238
Jawad AH, Ishak MAM, Farhan AM, Ismail K (2017) Response surface methodology approach for optimization of color removal and COD reduction of methylene blue using microwave-induced NaOH activated carbon from biomass waste. Desalin Water Treat 62:208–220
Ladeira NMB, Donnici CL, de Mesquita JP, Pereira FV (2021) Preparation and characterization of hydrogels obtained from chitosan and carboxymethyl chitosan. J Polym Res 28:335
Murali S, Kumar S, Koh J, Seena S, Singh P, Ramalho A, Sobral AJFN (2019) Bio-based chitosan/gelatin/Ag@ZnO bionanocomposites: synthesis and mechanical and antibacterial properties. Cellulose 26:5347–5361
Malek NNA, Mohammed IA, Reghioua A, Yousif E, Jawad AH (2020) Removal of reactive red 4 dye using chitosan-epichlorohydrin/ TiO2 nanocomposite: application of response surface methodology. Sci Lett 14(1):96–108
Malek NNA, Yousif E, Jawad AH (2020) Optimization of adsorption parameters for reactive red 4 (RR4) removal by cross-linked chitosan-epichlorohydrin using Box-Behnken design. Sci Lett 14(1):83–95
Saheed IO, Da OW, Suah FBM (2020) Chitosan modifications for adsorption of pollutants-a review. J Hazard Mater 408:124889
Aramesh N, Bagheri AR, Bilal M (2021) Chitosan-composite/hybrid biomaterials for adsorptive removal of dyes and underlying interaction mechanisms. Int J Biol Macromol 183:399–422
Reghioua A, Barkat D, Jawad AH, Abdulhameed AS, Khan MR (2021) Synthesis of Schiff’s base magnetic crosslinked chitosan-glyoxal/ZnO/Fe3O4 nanoparticles for enhanced adsorption of organic dye: Modeling and mechanism study. Sustain Chem Pharm 20:100379
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/TiO2 nanocomposite: application of response surface methodology. Desalin Water Treat 164:346–360
Liu S, Li Y, Yan J, Zhou Y, Liu C, Zuo Y, Liu D (2021) Effective removal of ruthenium (III) ions from wastewater by xanthate-modified cross-linked chitosan. J Environ Chem Eng 9(2):104818
Yuan D, Zhang W, Cui J, He L, Wang J, Yan C, Kou Y, Li J (2020) Facile fabrication of magnetic phosphorylated chitosan for the removal of Co(II) in water treatment: separation properties and adsorption mechanisms. Environ Sci Pollut Res 27:2588–2598
Reghioua A, Barkat D, Jawad AH, Abdulhameed AS, Rangabhashiyam S, Khan MR, ALOthman ZA (2021) Magnetic chitosan-glutaraldehyde/zinc oxide/Fe3O4 nanocomposite: optimization and adsorptive mechanism of remazol brilliant blue R dye removal. J Polym Environ 29: 3932–3947
Nga NK, Chau NTT, Viet PH (2020) Preparation and characterization of a chitosan/MgO composite for the effective removal of reactive blue 19 dye from aqueous solution. J Sci Adv Mater Dev 5(1):65–72
Dinh VP, Nguyen MD, Nguyen QH, Luu TT, Luu AT, Tap TD, Tan LV (2020) Chitosan-MnO2 nanocomposite for effective removal of Cr (VI) from aqueous solution. Chemosphere 257:127147
Sathiyavimal S, Vasantharaj S, Kaliannan T, Pugazhendhi A (2020) Eco-biocompatibility of chitosan coated biosynthesized copper oxide nanocomposite for enhanced industrial (Azo) dye removal from aqueous solution and antibacterial properties. Carbohydr Polym 241:116243
Wang Y, Cen C, Chen J, Fu L (2020) MgO/carboxymethyl chitosan nanocomposite improves thermal stability, waterproof and antibacterial performance for food packaging. Carbohydr Polym 236:116078
Haldorai Y, Shim JJ (2014) An efficient removal of methyl orange dye from aqueous solution by adsorption onto chitosan/MgO composite: A novel reusable adsorbent. Appl Surf Sci 292:447–453
Riyadh SM, Khalil KD, Aljuhani A (2018) Chitosan-MgO nanocomposite: One pot preparation and its utility as an ecofriendly biocatalyst in the synthesis of thiazoles and [1, 3, 4] thiadiazoles. Nanomaterials (Basel) 8(11):928
Zhang K, An Y, Zhang L, Dong Q (2012) Preparation of controlled nano-MgO and investigation of its bactericidal properties. Chemosphere 89(11):1414–1418
Reddy DHK, Lee SM (2013) Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv Colloid Interf Sci 201:68–93
Vieira RS, Beppu MM (2006) Interaction of natural and crosslinked chitosan membranes with Hg (II) ions. Colloids Surf A Physicochem Eng Asp 279(1–3):196–207
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater 404:179–189
Jawad AH, Mamat NH, Hameed BH, Ismail K (2019) Biofilm of cross-linked chitosan-ethylene glycol diglycidyl ether for removal of reactive red 120 and methyl orange: adsorption and mechanism studies. J Environ Chem Eng 7(2):102965
Pandey S, Tiwari S (2015) Facile approach to synthesize chitosan based biocomposite–characterization and cadmium (II) ion adsorption studies. Carbohydr Polym 134:646–656
Abdulhameed AS, Hum NNMF, Rangabhashiyam S, Jawad AH, Wilson LD, Yaseen ZM, ALOthman ZA (2021) Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator. J Environ Chem Eng 9(4):105530
Jawad AH, Abdulhameed AS, Wilson LD, Syed-Hassan SS, ALOthman ZA, Khan MR, Chin (2021) High surface area and mesoporous activated carbon from KOH-activated Dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. J Chem Eng 32:281–290
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. K vet akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124
Malek NNA, Jawad AH, Ismail K, Razuan R, ALOthman ZA (2021) Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: Adsorption parametric optimization. Int J Biol Macromol 189:464–476
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. Am Chem Soc 40(9):1361–1403
Frenudlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. URSS 12:327–356
Wang M, Day S, Wu Z, Wan X, Ye X, Cheng B (2021) A new type of porous Zn (II) metal-organic gel designed for effective adsorption to methyl orange dye. Colloids Surf A Physicochem Eng Asp 628
Ogunleye DT, Akpotu SO, Moodley B (2020) Adsorption of sulfamethoxazole and reactive blue 19 using graphene oxide modified with imidazolium based ionic liquid. Environ Technol Innov 17:100616
Vijayaraghavan K, Won SW, Yun YS (2009) Treatment of complex Remazol dye effluent using sawdust-and coal-based activated carbons. J Hazard Mater 167:790–796
Janaki V, Oh BT, Shanthi K, Lee KJ, Ramasamy AK, Kamala-Kannan S (2012) Polyaniline/chitosan composite: an eco-friendly polymer for enhanced removal of dyes from aqueous solution. Syn Met 162:974–980
Phan DN, Rebia RA, Saito Y, Kharaghani D, Khatri M, Tanaka T, Kim IS (2020) Zinc oxide nanoparticles attached to polyacrylonitrile nanofibers with hinokitiol as gluing agent for synergistic antibacterial activities and effective dye removal. J Ind Eng Chem 85:258–268
Nair V, Panigrahy A, Vinu R (2014) Development of novel chitosan–lignin composites for adsorption of dyes and metal ions from wastewater. Chem Eng J 254:491–502
Abbasi M (2017) Synthesis and characterization of magnetic nanocomposite of chitosan/SiO2/carbon nanotubes and its application for dyes removal. J Clean Prod 145:105–113
Chinoune K, Bentaleb K, Bouberka Z, Nadim A, Maschke U (2016) Adsorption of reactive dyes from aqueous solution by dirty bentonite. Appl Clay Sci 123:64–75
Mate CJ, Mishra S (2020) Synthesis of borax cross-linked Jhingan gum hydrogel for remediation of Remazol Brilliant Blue R (RBBR) dye from water: adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int J Boil Macromol 151:677–690
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: Adsorption and mechanism study. Int J Boil Macromol 142:732–741
Funding
The authors would like to thank the Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam for all facilities. The author (Zeid A. ALOthman) is grateful to the Researchers Supporting Project No. (RSP-2021/1), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Ali H. Jawad; Conceptualization, Methodology, Software, Supervision, Project administration, funding acquisition, Writing–Review & Editing. Ahmed Saud Abdulhameed; Validation, Data Curation, Writing–Original Draft. Rangabhashiyam S; Validation, Data Curation, Writing–Original Draft. Muhammad Ridwan; Conceptualization, Methodology, Project administration, funding acquisition. Zaher Mundher Yaseen; Validation, Data Curation. Zeid A. ALOthman; Validation, Data Curation. Lee D. Wilsom; Formal analysis, Writing–Original Draft.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jawad, A.H., Abdulhameed, A.S., Selvasembian, R. et al. Magnetic biohybrid chitosan-ethylene glycol diglycidyl ether/magnesium oxide/Fe3O4 nanocomposite for textile dye removal: Box–Behnken design optimization and mechanism study. J Polym Res 29, 207 (2022). https://doi.org/10.1007/s10965-022-03067-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03067-6