Abstract
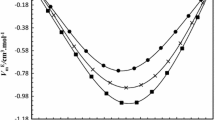
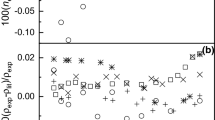
Experimental refractive index, \(n_{{\text{D}}}\), data for eleven binary systems of 2-diethylethanolamine (2-DEEA) + 1-propanol/1-butanol at different temperatures from T = 293.15 to 313.15 K and at atmospheric pressure, over the whole concentration range, are reported. The molar refractions (\(R_{{\text{m}}}\)), molecular radii \(\left( r \right)\), reduced molar free volumes \(\left( {{{V_{{\text{m}}} } \mathord{\left/ {\vphantom {{V_{{\text{m}}} } {R_{{\text{m}}} }}} \right. \kern-\nulldelimiterspace} {R_{{\text{m}}} }}} \right)\), internal pressure, \(\left( {P_{{\text{int}}} } \right)\) and the deviations of refractive indices \(\left( {\Delta n_{{\text{D}}} } \right)\), molar refraction \(\left( {\Delta R_{{\text{m}}} } \right)\), reduced molar free volumes \(\Delta \left( {{{V_{{\text{m}}} } \mathord{\left/ {\vphantom {{V_{{\text{m}}} } {R_{{\text{m}}} }}} \right. \kern-\nulldelimiterspace} {R_{{\text{m}}} }}} \right)\), and internal pressure \(\Delta \left( {P_{{\text{int}}} } \right)\) were also calculated from the experimental data. Nine mixing rules, viz. those of Arago and Biot, Newton, Heller, Gladstone and Dale, Eyring and John, Eykman, Lorentz and Lorentz, Weiner and Oster are used to predict the refractive indices of the binary liquid systems. Among all, the Weiner method (W) is in best agreement with the experimental results.




Similar content being viewed by others
References
Hossain, M.N., Rocky, H.M., Akhtar, S.: Density, refractive index, and sound velocity for the binary mixtures of tri-n-butyl phosphate and n-butanol between 303.15 K and 323.15 K. J. Chem. Eng. Data 61, 124–131 (2016)
Rahman, S.M., Saleh, A.M., Chowdhury, I.F., Ahmed, S.M., Rocky, H.M., Akhtar, S.: Density and viscosity for the solutions of 1-butanol with nitromethane and acetonitrile at 303.15 to 323.15 K. J. Mol. Liq. 190, 208–214 (2014)
Fucaloro, F.A.: Partial molar volumes from refractive index measurements. J. Chem. Educ. 79, 865 (2002)
Srivastava, R., Awasthi, A., Pandey, K.V., Awasthi, A.: Intermolecular interactions in binary mixtures of 2-diethylethanolamine with 1-propanol and 1-butanol at different temperatures. J. Chem. Thermodyn. 126, 11–21 (2018)
Pradhan, P., Roy, N.M.: Viscous synergy and antagonism, excess molar volume, isentropic compressibility and excess molar refraction of ternary mixtures containing tetrahydrofuran, methanol and six membered cyclic compounds at 298.15 K. Phys. Chem. Liq. 49, 286–301 (2011)
Kao, C.Y., Tu, H.C.: Densities, viscosities, refractive indices, and surface tensions for binary and ternary mixtures of 2-propanol, tetrahydropyran, and 2,2,4-trimethylpentane. J. Chem. Thermodyn. 43, 216–226 (2011)
Nain, K.A.: Ultrasonic and viscometric studies of molecular interactions in binary mixtures of formamide with ethanol, 1-propanol, 1,2-ethanediol and 1,2-propanediol at different temperatures. J. Mol. Liq. 140, 108–116 (2008a)
Francesconi, R., Ottani, S.: Correlation of density and refraction index for liquid binary mixtures containing polyglycols. Use of the group contributions in the Lorentz-Lorenz, Gladstone-Dale and Vogel equations to evaluate the density of mixtures. J. Mol. Liq. 133, 125–133 (2007)
Scholz, E.: Karl Fischer Titration. Springer, Berlin (1984)
Riddick, A.J., Bunger, B.W., Sakano, T.: Organic Solvents: Physical Properties and Methods of Purification. Wiley, New York (1986)
Vogel, I.A.: Text Book of Practical Organic Chemistry. Longman Green, London (1989)
Pandey, K.P., Awasthi, A., Awasthi, A.: Intermolecular interactions in binary mixtures of 2-chloroethanol with 2-dimethylaminoethanol and 2-diethylaminoethanol at different temperatures. Chem. Phys. 423, 119–126 (2013)
Bajic, M.D., Zivkovi, M.E., Serbanovi, P.S., Kijevcanin, L.M.: Experimental measurements and modelling of volumetric properties, refractive index and viscosity of selected binary systems with butylacetate at 288.15–323.15 K and atmospheric pressure. New UNIFAC-VISCO interaction parameters. Thermochim. Acta 562, 42–55 (2013)
Mega, J.: Densities and refractive indices of pure alcohols as a function of temperature. J. Chem. Eng. Data 27, 312–317 (1982)
Singh, S., Aznar, M., Deenadayalu, N.: Densities, speeds of sound, and refractive indices for binary mixtures of 1-butyl-3-methylimidazolium methyl sulphate ionic liquid with alcohols at T = (298.15, 303.15, 308.15, and 313.15) K. J. Chem. Thermodyn. 57, 238–247 (2013)
Farhan, M., Awwad, M.A.: Relative permittivities, refractive indices, and densities of dihydrofuran-2(3H)-one + butan-1-ol and + butan-2-ol at T = 293.15, 298.15, 303.15, and 313.15) K. J. Chem. Eng. Data 55, 1035–1038 (2010)
Yang, K.S., Peng, J.S., Huang, H.J., Fan, Q.L., Yang, X.F.: A study of densities and excess volumes in the (γ-butyrolactone + aromatic hydrocarbon) system at various temperatures. J. Chem. Thermodyn. 39, 773–780 (2007)
Chmielewska, A., Zurada, M., Klimaszewski, K., Bald, A.: Dielectric properties of methanol mixtures with ethanol, isomers of propanol, and butanol. J. Chem. Eng. Data 54, 801–806 (2009)
Saravanakumar, K., Baskaran, R., Kubendran, R.T.: Thermophysical properties of acetophenone with N, N-dimethylethanolamine or with N, N-diethylethanolamine at temperatures of (303.15, 313.15 and 323.15) K and pressure of 0.1 MPa. J. Solution Chem. 40, 955–967 (2011)
CRC Handbook of Chemistry and Physics. 71st edn. CRC Press Inc., Boca Raton (1990–1991)
Rodrıguez, A., Canosa, J., Tojo, J.: Physical properties of binary mixtures (dimethyl carbonate + alcohols) at several temperatures. J. Chem. Eng. Data 46, 1476–1486 (2001)
Zivkovi, N., Serbanovi, S., Kijevcanin, M., Zivkovi, E.: Volumetric properties, viscosities, and refractive indices of the binary systems 1-butanol + PEG 200, + PEG 400, and + TEGDME. Int. J. Thermophys. 34, 1002–1020 (2013)
Fialkov, Y.Y., Fenerly, N.G.: Use of volume properties in the physicochemical analysis of binary liquid systems. Russ. J. Inorg. Chem. 9, 1205–1209 (1964)
Fialkov, Y.Y.: Molar-additive properties in physicochemical analysis of binary liquid systems. Russ. J. Phys. Chem. 41, 398–400 (1967)
Brocos, P., Pineiro, A., Bravo, R., Amigo, A.: Refractive indices, molar volumes and molar refractions of binary liquid mixtures: concepts and correlations. Phys. Chem. Chem. Phys. 5, 550–557 (2003)
Hirschfelder, J.O., Curtiss, C.F., Bird, R.B.: Molecular Theory of Gases and Liquids. Wiley, New York (1964)
Arago, J.F.D., Biot, B.J.: Memory on the affinities of bodies for light and particularly on the strengths of the different refractive gas. Mem. Acad. France. 15, 7–11 (1806)
Dale, D.: Gladstone, F: on some optical properties of phosphorus Philos. Trans. R. Soc. Lond. 148, 887–890 (1858)
Gupta, M., Vibhu, I., Shukla, P.J.: Refractive index, molar refraction deviation and excess molar volume of binary mixtures of 1,4-dioxane with carboxylic acids. Phys. Chem. Liq. 48, 415–427 (2010)
Eyring, H., John, M.S.: Significant Liquid Structures. Wiley, New York (1969)
Lorentz, A.H.: Theory of Electrons, Leipzig (1906)
Heller, J.W.: Remarks on refractive index mixture rules. J. Phys. Chem. 69, 1123–1129 (1965)
Prigogine, I.: Molecular Theory of Solutions. North Holland, Amsterdam (1957)
Oster, G.: The scattering of light and its applications to chemistry. Chem. Rev. 43, 319–365 (1948)
Weiner, O.: Refractive index mixing rule in higher alkanes and alkanol systems. Berichte 62, 256–260 (1910)
Billah, M.M., Rocky, H.M.M., Hossen, I., Hossain, N., Akhtar, S.: Densities, viscosities, and refractive indices for the binary mixtures of tri-n-butyl phosphate (TBP) with toluene and ethylbenzene between (303.15 and 323.15) K. J. Mol. Liq. 265, 611–620 (2018)
Beguma, K.S., Ratna, A.S., Clarke, J.R., Ahmed, S.M.: Excess molar volumes, refractive indices and transport properties of aqueous solutions of poly(ethylene glycol)s at (303.15–323.15) K. J. Mol. Liq. 202, 176–188 (2015)
Krishna, S.T., Narendra, K., Sankar, G.M., Nain, K.A., Munibhadrayya, B.: Thermodynamic, excess and optical studies on the intermolecular interactions of binary liquid mixtures of imidazolium based ILs. J. Chem. Therm. 98, 262–271 (2016)
Marcus, Y.: Introduction to Liquid State Chemistry. Wiley, New York (1977)
Ali, A., Ansari, S., Nain, K.A.: Densities, refractive indices and excess properties of binary mixtures of dimethylsulphoxide with some poly(ethylene glycol)s at different temperatures. J. Mol. Liq. 178, 178–184 (2013)
Deetlefs, M., Seddon, K.R., Shara, M.: Predicting physical properties of ionic liquids. Phys. Chem. Chem. Phys. 8, 642–649 (2006)
Pineiro, A., Brocos, P., Amigo, A., Pintos, M., Bravo, R.: Prediction of excess volumes and excess surface tensions from experimental refractive indices. Phys. Chem. Liq. 38, 251–260 (2000)
Nain, K.A.: Refractive indices and deviations in refractive indices for binary mixtures of formamide + 1-butanol, + 2-butanol, + 1,3-butanediol, and + 1,4-butanediol at temperatures from (293.15 to 318.15) K. J. Chem. Eng. Data 53, 1208–1210 (2008b)
Ali, A., Tariq, M.: Deviations in refractive index parameters and applicability of mixing rules in binary mixtures of benzene + 1,2-dichloroethane at different temperatures. Chem. Eng. Commun. 195, 43–56 (2007)
Garcia, B., Alcalde, R., Aparicio, S., Leal, M.J.: The N-methylpyrrolidone–(C1–C10) alkan-1-ols solvent systems. Phys. Chem. Chem. Phys. 4, 1170–1177 (2002)
Aminabhavi, M.T., Patil, B.V., Banerjee, K., Balundgi, H.R.: Thermodynamic interactions in binary mixtures of styrene with n-alkanes at 298.15 K. Bull. Chem. Soc. Jpn. 72, 1187–1195 (1999)
Acknowledgements
The author Vikash Verma is thankful to the University Grants Commission, New Delhi, India providing financial support (Ref. No.—20/12/2015(ii) EU-V).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pandey, V.K., Verma, V., Srivastava, R. et al. Refractive Indices and Their Related Properties for Binary Mixtures Containing 2-Diethylethanolamine with 1-Propanol and 1-Butanol. J Solution Chem 49, 1459–1472 (2020). https://doi.org/10.1007/s10953-020-01032-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01032-9