Abstract
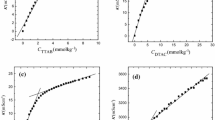
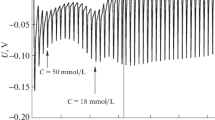
Interactions of the fluoroquinolone antibiotic drug levofloxacin hemihydrate (LFH) with tetradecyltrimethylammonium bromide (TTAB) were studied in water and in the presence of additives sodium chloride (NaCl) and ethanol (C2H5OH) at 303.15 to 323.15 K with an interval of 5 K. Only one critical micelle concentration (c*) was obtained for TTAB in water. However, three different critical micelle concentrations \( c_{1}^{*} \) (first break), \( c_{2}^{*} \) (second break, and \( c_{3}^{*} \) (third break) were observed for LFH + TTAB mixtures in all the cases. The c* values for (TTAB + LFH) mixtures in the presence of sodium chloride (NaCl) were lower than those in water. On the contrary, the micellization of TTAB was disfavored by the presence of ethanol and the values of c* for (TTAB + LFH) mixed systems were higher than those in water. The micellization was almost enthalpy controlled for (TTAB + LFH) systems in water, and in the aqueous C2H5OH and NaCl solutions but at some temperatures it became both entropy and enthalpy controlled. The standard state Gibbs energy of transfer (\( \Delta_{\text{tr}} G_{\text{m}}^{\circ} \)), enthalpy of transfer (\( \Delta_{\text{tr}} H_{\text{m}}^{\circ} \)) and entropy of transfer (\( \Delta_{\text{tr}} S_{\text{m}}^{\circ} \)) were also determined and are discussed in detail. TTAB and LFH + TTAB systems exhibit excellent enthalpy–entropy compensation in all the cases.






Similar content being viewed by others
References
Rosen, M.J.: Surfactants and Interfacial Phenomenon, 3rd edn. The City University of New York, Wiley (2004). ISBN 0-471-47818-0
Khan, F., Siddiqui, U.S., Rub, M.A., Khan, I.A., Kabir-ud-Din: Micellization behavior of butanediyl-1,4-bis(dimethyldodecylammonium bromide) gemini surfactant in presence of organic additives. J. Dispers. Sci. Tech. 36, 83–93 (2015)
Azum, N., Rub, M.A., Asiri, A.M.: Interaction of triblock-copolymer with cationic gemini and conventional surfactants: a physicochemical study. J. Disp. Sci. Technol. 38, 1785–1791 (2017)
Rub, M.A., Azum, N., Khan, F., Asiri, A.M.: Aggregation of sodium salt of ibuprofen and sodium taurocholate mixture in different media: a tensiometry and fluorometry study. J. Chem. Thermodyn. 121, 199–210 (2018)
Rub, M.A., Azum, N., Khan, F., Asiri, A.M.: Surface, micellar, and thermodynamic properties of antidepressant drug nortriptyline hydrochloride with TX-114 in aqueous/urea solutions. J. Phys. Org. Chem. 30, e3676 (2017)
Rahman, M., Khan, M.A., Rub, M.A., Hoque, M.A.: Effect of temperature and salts on the interaction of cetyltrimethylammonium bromide with ceftriaxone sodium trihydrate drug. J. Mol. Liq. 223, 716–724 (2016)
Buckingham, L.E., Balasubramanian, M., Emanuele, R.M., Clodfelter, K.E., Coon, J.S.: Comparison of Solutol HS 15, Cremophor EL and novel ethoxylated fatty acid surfactants as multidrug resistance modification agents. Int. J. Cancer 62, 436–442 (1995)
Nerurkar, M.M., Ho, N.F., Burton, P.S., Vidmar, T.J., Borchardt, R.T.: Mechanistic roles of neutral surfactants on cocurrent polarized and passive membrane transport of a model peptide in Caco-2 cells. J. Pharm. Sci. 86, 813–821 (1997)
Fendler, J.H., Fendler, E.J.: Catalysis in Micellar and Macromolecular Systems. Academic Press, New York (1975). ISBN 978-0-12-252850-7
Attwood, D., Florence, A.T.: Surfactant Systems; Their Chemistry, Pharmacy and Biology. Chapman & Hall, New York (1983). https://doi.org/10.1007/978-94-009-5775-6
Kumar, D., Hidayathulla S., Rub, M.A.: Association behavior of a mixed system of the antidepressant drug imipramine hydrochloride and dioctyl sulfosuccinate sodium salt: Effect of temperature and salt. J. Mol. Liq. 271, 254–264 (2018)
Kumar, D., Rub, M.A.: Aggregation behavior of amphiphilic drug promazine hydrochloride and sodium dodecylbenzenesulfonate mixtures under the influence of NaCl/urea at various concentration and temperatures. J. Phys. Org. Chem. 29, 394–405 (2016)
Chauhan, S., Sharma, V., Pathania, L.: Probing effect of maltodextrin on micellar properties of bile salts at varying temperature. J. Mol. Liq. 269, 304–314 (2018)
McManus, P.S., Stockwell, V.O., Sundin, G.W., Jones, A.L.: Antibiotic use in plant agriculture. Ann. Rev. Phytopathol. 40, 443–465 (2002)
Hoque, M.A., Khan, M.A., Hossain, M.D.: Interaction of cefalexin monohydrate with cetyldimethylethylammonium bromide. J. Chem. Thermodyn. 60, 71–75 (2013)
Molla, M.R., Rub, M.A., Ahmad, A., Hoque, M.A.: Interaction between tetradecyltrimethylammonium bromide and benzyldimethylhexadecylammonium chloride in aqueous/urea solution at various temperatures: an experimental and theoretical investigation. J. Mol. Liq. 238, 62–70 (2017)
Kumar, D., Rub, M.A.: Effect of anionic surfactant and temperature on micellization behavior of promethazine hydrochloride drug in absence and presence of urea. J. Mol. Liq. 238, 389–396 (2017)
Kumar, K., Patel, B.S., Chauhan, S.: Conductivity and fluorescence studies on the micellization properties of sodium cholate and sodium deoxycholate in aqueous medium at different temperatures: effect of selected amino acids. J. Chem. Thermodyn. 82, 25–33 (2015)
Azum, N., Rub, M.A., Asiri, A.M.: Interaction of antipsychotic drug with novel surfactants: micellization and binding studies. Chin. J. Chem. Eng. 26, 566–573 (2018)
Ropers, M.H., Czichocki, G., Brezesinski, G.: Counterion effect on the thermodynamics of micellization of alkyl sulfates. J. Phys. Chem. B 107, 5281–5288 (2003)
Diamant, H., Andelman, D.: Kinetics of surfactant adsorption at fluid–fluid interfaces. J. Phys. Chem. 100, 13732–13742 (1996)
Caetano, W., Tabak, M.: Interaction of chlorpromazine and trifluoperazine with anionic sodium dodecyl sulphate (SDS) micelles: electronic absorption and fluorescence studies. J. Colloid Interface Sci. 225, 69–81 (2000)
Minatti, E., Zanette, D.: Salt effects on the interaction of poly(ethylene oxide) and sodium dodecyl sulfate measured by conductivity. Colliods Surf. A 113, 237–246 (1996)
Khan, F., Rub, M.A., Azum, N., Asiri, A.M.: Mixtures of antidepressant amphiphilic drug imipramine hydrochloride and anionic surfactant: micellar and thermodynamic investigation. J. Phys. Org. Chem. 31, e3812 (2018)
Rub, M.A., Asiri, A.M., Khan, J.M., Khan, F., Khan, R.H., Kabir-ud-Din: A study of interaction between antidepressant drug nortriptyline hydrochloride with gelatin. J. Taiwan Inst. Chem. Eng. 45, 2068–2074 (2014)
Chakraborty, T., Chakraborty, I., Ghosh, S.: Sodium carboxymethylellulose–CTAB interaction: a detailed thermodynamic study of polymer–surfactant interaction with opposite charges. Langmuir 22, 9905–9913 (2006)
Ruiz, C.C.: Thermodynamics of micellization of tetradecyltrimethylammonium bromide in ethylene glycol ± water binary mixtures. Colloid Polym. Sci. 277, 701–707 (1999)
Ruiz, C.C., Aguiar, J.: Mixed micellization of octaoxyethylenemonododecyl ether and n-alkyltrimethylammonium bromides. Colloids Surf. A 224, 221–230 (2003)
Garcı́a-Mateos, I., Pérez, S., Velázquez, M.M.: Interaction between cetyl pyridinium chloride and water-soluble polymers in aqueous solutions. J. Colloid Interface Sci. 194, 356–363 (1997)
Chauhan, S., Kaur, M., Rana, D.S., Chauhan, M.S.: Volumetric analysis of structural changes of cationic micelles in the presence of quaternary ammonium salts. J. Chem. Eng. Data 61, 3770–3778 (2016)
Pérez-Rodríguez, M., Prieto, G., Rega, C., Varela, L.M., Sarmiento, F., Mosquera, V.: A comparative study of the determination of the critical micelle concentration by conductivity and dielectric constant measurements. Langmuir 14, 4422–4426 (1998)
Tsubone, K.Y., Arakawa, Y., Rosen, M.J.: Structural effects on surface and micellar properties of alkanediyl-alpha, omega-bis(sodium N-acyl-beta-alaninate) gemini surfactants. J. Colloid Interface Sci. 262, 516–524 (2003)
Carale, T.R., Pham, Q.T., Blankschtein, D.: Salt effects on intramicellar interactions and micellization of nonionic surfactants in aqueous solutions. Langmuir 10, 109–121 (1994)
Hoque, M.A., Patoary, M.O.F., Rashid, M.M., Molla, M.R., Rub, M.A.: Physico-chemical investigation of the mixed micelle formation between tetradecyltrimethylammonium bromide and dodecyltrimethylammonium chloride in water and aqueous solution of sodium chloride. J. Solution Chem. 46, 682–703 (2017)
Zhang, H., Zhu, Y., Zhang, K., Hou, T., Liu, H., Cui, M., Li, G., Yu, L.: Studies on the CMC and thermodynamic functions of a cationic surfactant in DMF/long-chain alcohol systems using a microcalorimetric method. J. Solution Chem. 38, 187–198 (2009)
Mukerjee, P., Korematsu, K., Okawauchi, M., Sugihara, G.: Effect of temperature on the electrical conductivity and the thermodynamics of micelle formation of sodium perfluorooctanoate. J. Phys. Chem. 89, 5308–5312 (1985)
Ray, A., Nemethy, G.: Micelle formation by nonionic detergents in water–ethylene glycol mixtures. J. Phys. Chem. 75, 809–815 (1971)
Sidim, T., Acar, G.: Alcohols effect on critical micelle concentration of polysorbate 20 and cetyltrimethyl ammonium bromide mixed solutions. J. Surfact. Deterg. 16, 601–607 (2013)
Akhter, M.S.: Effect of solubilization of alcohols on critical micelle concentration of non-aqueous micellar solutions. Colloids Surf. A 157, 203–210 (1999)
Khan, F., Rub, M.A., Azum, N., Kumar, D., Asiri, A.M.: Interaction of an amphiphilic drug and sodium bis(2-ethylhexyl)sulfosuccinate at low concentrations in the absence and presence of sodium chloride. J. Solution Chem. 44, 1937–1961 (2015)
Mata, J., Varade, D., Bahadur, P.: Aggregation behaviour of quaternary salt based cationic surfactants. Thermochim. Acta 428, 147–155 (2005)
Kim, H.U., Lim., K.H.: A model on the temperature dependence of critical micelle concentration. Colloids Surf. A 235, 121–128 (2004)
Rahman, M., Khan, M.A., Rub, M.A., Hoque, M.A., Asiri, A.M.: Investigation of the effect of various additives on the clouding behavior and thermodynamics of polyoxyethylene (20) sorbitan monooleate in absence and presence of ceftriaxone sodium trihydrate drug. J. Chem. Eng. Data 62, 1464–1474 (2017)
Jalali, F., Shamsipur, M., Alizadeh, N.: Conductivity study of the thermodynamics of micellization of 1-hexadecylpyridinium bromide in (water + cosolvent). J. Chem. Thermodyn. 32, 755–765 (2000)
Owoyomi, O., Ige, J., Soriyan, O.O.: Thermodynamics of n-alkyltriphenylphosphnium bromides: a conductometric study. Chem. Sci. J. 25, 1–13 (2011)
Vamvaca, K., Jelesarov, I., Hilvert, D.: Kinetics and thermodynamics of ligand binding to a molten globular enzyme and its native counterpart. J. Mol. Liq. 382, 971–977 (2008)
Azum, N., Rub, M.A., Asiri, A.M.: Micellization and interfacial behavior of the sodium salt of ibuprofen-BRIJ-58 in aqueous/brine solutions. J. Solution Chem. 45, 791–803 (2016)
Rub, M.A., Azum, N., Asiri, A.M.: Binary mixtures of sodium salt of ibuprofen and selected bile salts: interface, micellar, thermodynamic, and spectroscopic study. J. Chem. Eng. Data 62, 3216–3228 (2017)
Nusselder, J.J.H., Engberts, J.B.F.N.: Toward a better understanding of the driving force for micelle formation and micellar growth. J. Colloid Interface Sci. 148, 353–361 (1992)
Rub, M.A., Azum, N., Khan, S.B., Khan, F., Asiri, A.M.: Physicochemical properties of amphiphilic drug and anionic surfactant mixtures: experimental and theoretical approach. J. Disp. Sci. Technol. 36, 521–531 (2015)
Rub, M.A., Khan, F., Kumar, D., Asiri, A.M.: Study of mixed micelles of promethazine hydrochloride (PMT) and nonionic surfactant (TX-100) mixtures at different temperatures and compositions. Tenside Surf. Deterg. 52, 236–244 (2015)
Kumar, D., Rub, M.A.: Effect of sodium taurocholate on aggregation behavior of amphiphilic drug solution. Tenside Surf. Deterg. 52, 464–472 (2015)
Rub, M.A., Asiri, A.M., Kumar, D., Azum, N., Khan, F.: Temperature dependant mixed micellization behavior of a drug–AOT mixture in an aqueous medium. Acta Phys. Chim. Sin. 30, 699–707 (2014)
Chen, L.J., Lin, S.Y., Huang, C.C., Chen, E.M.: Temperature dependence of critical micelle concentration of polyoxyethylenated non-ionic surfactants. Colloids Surf. A 135, 175–181 (1998)
Nalwa, H.S.: Handbook of Surfaces and Interfaces of Materials, vol. 3, p. 405. Academic Press (2001). ISBN: 978-0-12-513910-6
Khan, F., Sheikh, M.S., Rub, M.A., Azum, N., Asiri, A.M.: Antidepressant drug amitriptyline hydrochloride (AMT) interaction with anionic surfactant sodium dodecyl sulfate in aqueous/brine/urea solutions at different temperatures. J. Mol. Liq. 222, 1020–1030 (2016)
Rub, M.A., Sheikh, M.S., Khan, F., Khan, S.B., Asiri, A.M.: Bile salts aggregation behavior at various temperatures under the influence of amphiphilic drug imipramine hydrochloride in aqueous medium. Z. Phys. Chem. 228, 747–767 (2014)
Vamvaca, K., Jelesarov, I., Hilvert, D.: Kinetics and thermodynamics of ligand binding to a molten globular enzyme and its native counterpart. J. Mol. Biol. 382, 971–977 (2008)
Jelesarov, I., Bosshard, H.R.: Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J. Mol. Recognit. 12, 3–18 (1999)
Shivaji, S.K., Rakshit, A.K.: Investigation of the properties of decaoxyethylene n-dodecyl ether, C12E10, in the aqueous sugar-rich region. J. Surf. Deterg. 7, 305–316 (2004)
Rakshit, A.K., Sharma, B.: The effect of amino acids on the surface and thermodynamic properties of poly[oxyethylene(10)]lauryl ether in aqueous solution. Colloid Polym. Sci. 281, 45–51 (2003)
Jha, R., Ahluwalia, J.C.: Thermodynamics of micellization of some decyl poly(oxyethyleneglycol) ether in aqueous urea solution. J. Chem. Soc. Faraday Trans. 89, 3465–3469 (1993)
Chen, L.J., Lin, S.Y., Huang, C.C.: Effect of hydrophobic chain length of surfactants on enthalpy–entropy compensation of micellization. J. Phys. Chem. 102, 4350–4356 (1998)
Lumry, R., Rajender, S.: Enthalpy–entropy compensation phenomena in water solutions of proteins and small molecules: a ubiquitous properly of water. Biopolymers 9, 1125–1127 (1970)
Sugihara, G., Hisatomi, M.: Enthalpy–entropy compensation phenomenon observed for different surfactants in aqueous solution. J. Colloid Interface Sci. 219, 31–36 (1999)
Acknowledgements
The authors are thankful to The ACME Laboratories Ltd., BD for providing the standard sample of LFH as a gift item to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoque, M.A., Molla, M.R., Amin, M.R. et al. Investigation of the Effect of Temperature, Salt and Solvent Composition on the Micellization Behavior of Tetradecyltrimethylammonium Bromide in the Presence of the Antibiotic Drug Levofloxacin Hemihydrate. J Solution Chem 48, 105–124 (2019). https://doi.org/10.1007/s10953-019-00850-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-019-00850-w