Abstract
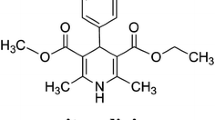
Solubilities of two calcium channel blockers, nifedipine and lacidipine, were measured in supercritical carbon dioxide at T = (313, 323, and 333) K over the pressure range (12.0–36.0) MPa using a dynamic-analytical apparatus. The solubility values obtained are in the range of (0.18–7.05) × 10−5 mol·mol−1. The solubilities of the two solids show similar trends with a crossover region of the respective isotherms in the range 18.0–21.0 MPa. The experimental solubility data were correlated with several different models. The semi-empirical density-based models provided satisfactory correlation results with AARD values lower than 10%. According to the results of the Méndez-Santiago and Teja models, the measured solid solubility data are quite consistent at all experimental conditions, which indicates the reliability of the data. The compressed gas model of the Peng–Robinson equation of state, combined with the two parameter van der Waals mixing rule (PR-EoS-VDW2) model, gives better correlation results than the PR-EoS-VDW1 model. The expanded liquid model based on Scatchard–Hildebrand regular solution theory can be used for solubility prediction, but the correlation results for nifedipine and lacidipine by the model are inferior to the compressed gas models in this work.







Similar content being viewed by others
Abbreviations
- T :
-
Temperature (in K), Eq. 2
- s :
-
Solubility of the solute (in kg·m−3), Eq. 2
- p :
-
Pressure (in MPa), Eq. 3
- p ref :
-
Reference pressure p ref = 0.1 MPa, Eq. 3
- y 2 :
-
Mole fraction of the solute in supercritical solution (in mol·mol−1), Eq. 5
- p sat2 :
-
The saturation pressure of the solute at the given temperature (in MPa), Eq. 5
- \( v_{2}^{\text{s}} \) :
-
Molar volume of the solid solute (in cm3·mol−1), Eq. 5
- R :
-
Gas constant R = 8.3145 J·mol−1·K−1, Eq. 5
- a m :
-
Attraction parameter of the supercritical solution (in MPa·m3·mol−1), Eq. 6
- b m :
-
Repulsion parameter of the supercritical solution (in cm3·mol−1), Eq. 7
- a :
-
Attraction parameter (in MPa·m3·mol−1), Eq. 10
- b :
-
Repulsion parameter (in cm3·mol−1), Eq. 10
- v :
-
Molar volume of the supercritical phase (in cm3·mol−1), Eq. 10
- \( f_{2}^{\text{s}} \) :
-
Solid solute fugacity (in MPa), Eq. 12
- \( f_{2}^{\text{l}} \) :
-
Liquid solute fugacity (in MPa), Eq. 12
- \( \Delta c_{\text{p}} \) :
-
Difference between the heat capacities of the liquid the solid solute (in J·mol−1), Eq. 12
- \( \Delta H_{\text{m}} \) :
-
Enthalpy of fusion of solute (in J·mol−1), Eq. 13
- T m :
-
Melting temperature of solute (in K), Eq. 13
- \( v_{2}^{l} \) :
-
Liquid molar volume of solute (in cm3·mol−1), Eq. 15
- \( v_{1} \) :
-
Molar volume of the sc-CO2 (cm3·mol−1), Eq. 16
- \( y_{2}^{ \exp } \) :
-
Experimental solubility of the solute in sc-CO2 (in mol·mol−1), Eq. 19
- \( y_{2}^{\text{cal}} \) :
-
Calculated solubility of the solute in sc-CO2 (in mol·mol−1), Eq. 19
- N :
-
Number of solubility values, Eq. 19
- W :
-
Mass of drug loaded in the extractor (in g), Eq. 20
- F :
-
Mass flow rate of CO2 (in g·s−1), Eq. 20
- ρ :
-
Density of sc-CO2 (in kg·m−3), Eq. 2
- ρ ref :
-
Reference density ρ ref = 700 kg·m−3, Eq. 3
- \( \varphi_{2}^{\text{sat}} \) :
-
Fugacity coefficient of the pure solute at the saturation pressure, Eq. 5
- \( \varphi_{2} \) :
-
Fugacity coefficient of the solute in the supercritical phase under the experimental conditions (p, T), Eq. 5
- γ 2 :
-
The solute activity coefficient in the supercritical phase under the experimental condition (p, T), Eq. 12
- \( \delta_{1} \) :
-
Solubility parameter of sc-CO2 (in MPa0.5), Eq. 15
- \( \delta_{2} \) :
-
Solubility parameter of the solute (in MPa0.5), Eq. 15
- \( \phi_{1} \) :
-
Volume fraction of the solvent, Eq. 15
- \( \tau \) :
-
Contact time (in s), Eq. 20
References
Coelho, J.P., Naydenov, G.P., Yankov, D.S., Stateva, R.P.: Experimental measurements and correlation of the solubility of three primary amides in supercritical CO2: acetanilide, propanamide, and butanamide. J. Chem. Eng. Data 58, 2110–2115 (2013)
Li, H.R., Jia, D.D., Zhu, Q.Q., Shen, B.Q.: Determination, correlation and prediction of the solubilities of niflumic acid, clofenamic acid and tolfenamic acid in supercritical CO2. Fluid Phase Equilib. 392, 95–103 (2015)
Skerget, M., Knez, Z., Hrncic, M.K.: Solubility of solids in sub and supercritical fluids: A review. J. Chem. Eng. Data 56, 694–719 (2011)
Weinstein, R.D., Hanlon, W.H., Donohue, J.P., Simeone, M., Rozich, A., Muske, K.R.: Solubility of felodipine and nitrendipine in liquid and supercritical carbon dioxide by cloud point and UV spectroscopy. J. Chem. Eng. Data 52, 256–260 (2007)
Tomasko, D.L., Li, H., Liu, D., Han, X., Wingert, M.J., Lee, L.J., Koelling, K.W.: A review of CO2 applications in the processing of polymers. Ind. Eng. Chem. Res. 42, 6431–6456 (2003)
Yeo, S.D., Kiran, E.: Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 34, 287–308 (2005)
Nalawade, S.P., Picchioni, F., Janssen, L.P.B.M.: Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 31, 19–43 (2006)
Marrero, J., Gani, R.: Group-contribution based estimation of pure component properties. Fluid Phase Equilib. 183–184, 183–208 (2001)
Poling, B.E., Prausnitz, J.M., O’Connell, J.P.: The Properties of Gases and Liquids, 5th edn. McGraw-Hill, New York (2001)
Immirzi, A., Perini, B.: Prediction of density in organic crystals. Acta Crystallogr. A 33, 216–218 (1977)
Fedors, R.F.: A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 14, 147–154 (1974)
Knez, Z., Skerget, M., Sencar-Bozic, P., Rizner, A.J.: Solubility of nifedipine and nitrendipine in supercritical CO2. J. Chem. Eng. Data 40, 216–220 (1995)
Deiters, U.K.: In: Brunner, G. (ed.) Supercritical Fluids as Solvents and Reaction Media, pp. 185–209. Elsevier, Amsterdam (2004)
Chrastil, J.: Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 86, 3016–3021 (1982)
Bartle, K.D., Clifford, A.A., Jafar, S.A., Shilstone, G.F.: Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 20, 713–756 (1991)
Méndez-Santiago, J., Teja, A.S.: The solubility of solids in supercritical fluids. Fluid Phase Equilib. 158, 501–510 (1999)
Prausnitz, J.M., Lichtenthaler, R.N., Gomes de Azevedo, E.: Molecular Thermodynamics of Fluid Phase Equilibria, 2nd edn. Prentice Hall. Inc, Engelwood Cliffs, NJ (1986)
Neau, E., Garnier, S., Avaullee, L.: A consistent estimation of sublimation pressures using a cubic equation of state and fusion properties. Fluid Phase Equilib. 164, 173–186 (1999)
Li, H., Li, S., Shen, B.: Correlation and prediction of the solubilities of solid n-alkanes in supercritical carbon dioxide using the Carnahan–Starling–van der Waals model with a density-dependent parameter. Fluid Phase Equilib. 325, 28–34 (2012)
Johnston, K.P., Eckert, C.A.: An analytical Carnahan–Staring van der Waals model hydrocarbon solids in supercritical fluids. AIChE J. 27, 773–779 (1981)
Huang, F., Li, M., Lee, L.L., Starling, K.E., Chung, F.T.H.: An accurate equation of state for carbon dioxide. J. Chem. Eng. Jpn. 18, 490–496 (1985)
Weinstein, R.D., Grotzinger, L.L., Salemo, P., Omiatek, D.M., Bessel, C.A.: Solubility of several short-chain lithium dialkyldithiocarbamates in liquid and supercritical carbon dioxide. J. Chem. Eng. Data 50, 2088–2093 (2005)
Medina, I., Bueno, J.L.: Solubilities of 2-nitroanisole and 3-phenyl-1-propanol in supercritical carbon dioxide. J. Chem. Eng. Data 45, 298–300 (2000)
Foster, N.R., Gurdial, G.S., Yu, J.S.L., Liong, K.K., Tilly, K.D., Ting, S.S.T., Lee, J.H.: Significance of the crossover pressure in solid–supercritical fluid phase equilibria. Ind. Eng. Chem. Res. 30, 1955–1964 (1991)
Li, H., Jia, D., Liu, R., Shen, B.: Correlating and estimating the solubilities of solid organic compounds in supercritical CO2 based on the modified expanded liquid model and the group contribution method. Fluid Phase Equilib. 385, 10–24 (2014)
Acknowledgement
This work was supported by the National Natural Science Foundation of China (21106107, 21206077).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Meng, T., Jia, D. et al. Solubility of Nifedipine and Lacidipine in Supercritical CO2: Measurement and Correlation. J Solution Chem 46, 70–88 (2017). https://doi.org/10.1007/s10953-016-0550-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0550-2