Abstract
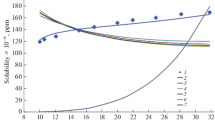
The solubilities of nitrendipine in supercritical carbon dioxide (ScCO2) and in the presence of 1, 3, 5, and 7 mol.% ethanol, were measured at 308, 318, and 328 K over a pressure range of 8–20 MPa. The experimental results reveal that the solubility increased with increasing pressure; the effect of temperature was more complex with or without cosolvent. The solubilities obtained in pure ScCO2 are relatively low, and they were successfully correlated by two semi-empirical models (Chrastil, Sung and Shim (SS) models). The solubility is increased significantly with ethanol as cosolvent, and these data were also correlated well using the modified Méndez-Santiago and Teja (M-ST) models.











Similar content being viewed by others
References
Weinstein, R.D., Hanlon, W.H., Donohue, J.P., Simeone, M., Rozich, A., Muske, K.R.: Solubility of felodipine and nitrendipine in liquid and supercritical carbon dioxide by cloud point and UV spectroscopy. J. Chem. Eng. Data 52, 256–260 (2007)
Ren, J., Zhan, S., Zhou, J., Zhang, M.: New equilibrium autoclave for determining solubility and melting point of solid solute in supercritical fluids. 1. Determination of solubility of levonorgestrel in supercritical carbon dioxide. Ind. Eng. Chem. Res. 51, 2451–2455 (2012)
Kopcak, U., Mohamed, R.S.: Caffeine solubility in supercritical carbon dioxide/co-solvent mixtures. J. Supercrit. Fluids 34, 209–214 (2005)
Li, J.L., Jin, J.S., Zhang, Z.T., Wang, Y.B.: Measurement and correlation of solubility of benzamide in supercritical carbon dioxide with and without cosolvent. Fluid Phase Equilibr. 307, 11–15 (2011)
Ting, S.S., Tomasko, D.L., Foster, N.R., Acnaughton, S.J.: Solubility of naproxen in supercritical carbon dioxide with and without cosolvents. Ind. Eng. Chem. Res. 32, 1471–1481 (1993)
Coelho, J.P., Mendonça, A.F., Palavra, A.F., Stateva, R.P.: On the solubility of three disperse anthraquinone dyes in supercritical carbon dioxide: new experimental data and correlation. Ind. Eng. Chem. Res. 50, 4618–4624 (2011)
Nejad, S., Mohammadikhah, R., Abolghasemi, H., Moosavian, M.A., Maragheh, M.G.: A novel equation of state (EOS) for prediction of solute solubility in supercritical carbon dioxide: experimental determination and correlation. Can. J. Chem. Eng. 87, 930–938 (2009)
Garlapati, C., Madras, G.: New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochim. Acta 500, 123–127 (2010)
Coutsikos, P., Magoulas, K., Kontogeorgis, G.M.: Prediction of solid–gas equilibria with the Peng–Robinson equation of state. J. Supercrit. Fluids 25, 197–212 (2003)
Tang, Z., Jin, J.S., Zhang, Z.T., Liu, H.T.: New experimental data and modeling of the solubility of compounds in supercritical carbon dioxide. Ind. Eng. Chem. Res. 51, 5515–5526 (2012)
Asiabi, H., Yamini, Y., Moradi, M.: Determination of sulfonylurea herbicides in soil samples via supercritical fluid extraction followed by nanostructured supramolecular solvent microextraction. J. Supercrit. Fluids 84, 20–28 (2013)
Reddy, S.N., Madras, G.: Mixture solubilities of nitrobenzoic acid isomers in supercritical carbon dioxide. J. Supercrit. Fluids 70, 66–74 (2012)
Chrastil, J.: Solubility of solids and liquids in supercritical gases. J. Phys. l Chem. 86, 3016–3021 (1982)
Méndez-Santiago, J., Teja, A.S.: The solubility of solids in supercritical fluids. Fluid Phase Equilibr. 158, 501–510 (1999)
Kumar, S.K., Johnston, K.P.: Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids 1, 15–22 (1988)
Sung, H.D., Shim, J.J.: Solubility of CI disperse red 60 and C.I. disperse blue 60 in supercritical carbon dioxide. J. Chem. Eng. Data 44, 985–989 (1999)
Bartle, K.D., Clifford, A.A., Jafar, S.A., Shilstone, G.F.: Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 20, 713–756 (1991)
Sparks, D.L., Estévez, L.A., Hernandez, R., Barlow, K., French, T.: Solubility of nonanoic (pelargonic) acid in supercritical carbon dioxide. J. Chem. Eng. Data 53, 407–410 (2008)
Jouyban, A., Chan, H.K., Foster, N.R.: Mathematical representation of solute solubility in supercritical carbon dioxide using empirical expressions. J. Supercrit. Fluids 24, 19–35 (2002)
National Institute of Standards and Technology.: Thermophysical Properties of Fluid System. http://webbook.nist.gov/chemistry/fluid/. Accessed Feb 09, (2011)
Xu, Q., Han, B.X., Yan, H.K.: Density of supercritical CO2-tetrahydrofuran binary mixture and the partial molar volume of the cosolvent. Chin. J. Chem. 16, 414–420 (1998)
Li, Y., Ning, Y., Jin, J., Zhang, Z.: Solubility of 3-aminobenzoic acid in supercritical carbon dioxide modified by ethanol. J. Chem. Eng. Data 58, 2176–2180 (2013)
Coelho, J.P., Naydenov, G.P., Yankov, D.S., Stateva, R.P.: Experimental measurements and correlation of the solubility of three primary amides in supercritical CO2: acetanilide, propanamide, and butanamide. J. Chem. Eng. Data 58, 2110–2115 (2013)
Hezave, A.Z., Rajaei, H., Lashkarbolooki, M., Esmaeilzadeh, F.: Analyzing the solubility of fluoxetine hydrochloride in supercritical carbon dioxide. J. Supercrit. Fluids 73, 57–62 (2013)
Jin, J.S., Ning, Y.Y., Hu, K., Wu, H., Zhang, Z.T.: Solubility of p-nitroaniline in supercritical carbon dioxide with and without mixed cosolvents. J. Chem. Eng. Data 58, 1464–1469 (2013)
Fan, J., Hou, Y., Wu, W., Zhang, J., Ren, S., Chen, X.: Levulinic acid solubility in supercritical carbon dioxide with and without ethanol as cosolvent at different temperatures. J. Chem. Eng. Data 55, 2316–2321 (2010)
Li, J.L., Jin, J.S., Zhang, Z.T., Pei, X.M.: Solubility of p-toluenesulfonamide in pure and modified supercritical carbon dioxide. J. Chem. Eng. Data 54, 1142–1146 (2009)
Acknowledgments
The authors acknowledge the funding provided by the Natural Science foundation of China (Project 21176032).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhan, S., Cui, L., Zhao, Q. et al. Measurement and Correlation of Solubility of Nitrendipine in Supercritical Carbon Dioxide With and Without Ethanol Cosolvent. J Solution Chem 44, 1–15 (2015). https://doi.org/10.1007/s10953-014-0262-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0262-4