Abstract
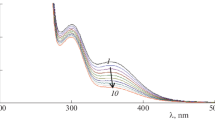
The kinetics of the oxidation of ruthenium(III) (Ru(III)) and osmium(VIII) (Os(VIII)) catalyzed oxidation of d-glucose (d-Glu) by silver(III) periodate complex (DPA) in aqueous alkaline medium at 298 K and constant ionic strength 0.003 mol·dm−3 was studied spectrophotometrically. The reaction between d-Glu and DPA in alkaline medium exhibits 1:2 stoichiometry in both catalyzed reactions (d-Glu:DPA). The main products were identified as D-arabinonic acid and formic acid by spot tests, GC–MS spectra and chromatographic techniques. The reaction orders with respect to various species concentrations were determined. Also, the active species of catalyst and oxidant have been identified. Probable mechanisms were proposed. The activation parameters with respect to the slow step of the mechanism were computed and discussed and thermodynamic quantities were also calculated. It has been observed that the catalytic efficiency for the present reaction is in the order Os(VIII) > Ru(III).





Similar content being viewed by others
References
Venkatakrishna, K., Rao, J.P.: Kinetics and mechanism of oxidation of pyrimidine nucleobases by diperiodatoargentate(III) in aqueous alkaline medium. Transition Met. Chem. 20, 344–346 (1995)
Sethuram, B.: Some Aspects of Electron Transfer Reactions Involving Organic Molecules, p. 151. Allied Publishers (P) Ltd, New Delhi (2003)
Jaiswal, P.K., Yadava, K.L.: Silver(III) as an oxidative titrant: Determination of some sugars, carboxylic acids and inorganic ions. Talanta 17, 236–238 (1970)
Jaiswal, P.K.: Ditelluratoargentate(III) and ditelluratocuprate(III) as reagent in microanalysis of cellobiose, melibiose, mixture of some aliphatic acids, and formaldehyde and formic acid. Analyst 1, 503–506 (1972)
Jayaprakash Rao, P., Sethuram, B., Navaneeth Rao, T.: Kinetics of oxidative deamination of some amino acids by diperiodatoargentate(III) in alkaline medium. React. Kinet. Catal. Lett. 29, 288–296 (1985)
VenkataKrishna, K., Jayaprakash Rao, P.: Kinetics and mechanism of oxidation of pyrimidine nucleobases by diperiodatoargentate(III) in aqueous alkaline medium. Indian J. Chem. 37A, 1106–1109 (1998)
Kumar, A., Kumar, P., Ramamurthy, P.: Kinetics of oxidation of glycine and related substrates by diperiodatoargentate(III). Polyhedron 18, 773–780 (1999)
Kumar, A., Kumar, P.: Kinetics and mechanism of oxidation of nitrilotriacetic acid by diperiodatoargentate(III). J. Phys. Org. Chem. 12, 79–82 (1999)
Kumar, A., Vaishali, P., Ramamurthy, P.: Kinetics and mechanism of oxidation of ethylenediamine and related compounds by diperiodatoargentate(III). Int. J. Chem. Kinet. 32, 286–293 (2000)
Das, A.K.: Kinetic and mechanistic aspects of metal ion catalysis in cerium(IV) oxidation. Coord. Chem. Rev. 213, 307–325 (2001)
Agrawal, M.C., Upadhyay, S.K.J.: Osmium(VIII) as catalyst in some redox reactions. Sci. Ind. Res. 42, 508–510 (1983)
Hugar, G.H., Nandibewoor, S.T.: Kinetics of osmium(VIII) catalysis of periodate oxidation of DMF in aqueous alkaline medium. Transition Met. Chem. 19, 215–217 (1994)
Veerasomaiah, P., Bal Reddy, K., Sethuram, B., Navaneeth Rao, T.: Role of osmium tetroxide in oxidation of 2-propanol in presence and absence of different one and two-equivalent oxidants in aqueous alkaline medium. Indian J. Chem. 26A, 402–406 (1987)
Khan, Z.: Kabir-ud-Din: kinetics and mechanism of oxidation d-glucose by chromium(VI) in perchloric acid. Indian J. Chem. 39A, 522–527 (2000)
Singh, A.K., Shalini, S., Srivastava, J., Srivastava, R., Singh, P.: Studies in kinetics and mechanism of oxidation of d-glucose and d-fructose by alkaline solution of potassium iodate in the presence of Ru(III) as homogeneous catalyst. J. Mol. Catal. A. Chem. 278, 72–81 (2007)
Saxena, O.C.: New titrimetric microdetermination of osmium. Microchem. J. 12, 609–611 (1967)
Reddy, C.S., Vijaya, K.T.: Kinetics and mechanism of uncatalyzed and ruthenium(III) catalyzed oxidation of allyl alcohol by N-bromosuccinimide in aqueous alkaline medium. Indian J. Chem. 34A, 615–622 (1995)
Kamble, D.L., Chougale, R.B., Nandibewoor, S.T.: Kinetics of oxidation of chromium(III) by N-bromosuccinimide in aqueous alkaline medium. Indian J. Chem. 35A, 865–869 (1996)
Panigrahi, G.P., Misro, P.K.: Kinetics and mechanism of oxidation of osmium(VIII) catalysed oxidation of unsaturated acids by sodium periodate. Indian J. Chem. 15A, 1066–1069 (1977)
Cohen, G.L., Atkinson, G.: The chemistry of argentic oxide. The formation of a silver(III) complex with periodate in basic solution. Inorg. Chem. 3, 1741–1743 (1964)
Jeffery, G.H., Bassett, J., Mendham, J., Denney, R.C.: Vogel’s Textbook of Quantitative Chemical Analysis, 5th edn., pp. 467, 391. Longmans Singapore Publishers Pvt Ltd., Singapore (1996)
Feigl, F.: Spot Tests in Organic Analysis, p. 368. Elsevier, New York (1960)
Gutbauer, F.: Chromatographic behaviour and chemical structure: I. Thin-layer chromatography of aliphatic acids. J. Chromatogr. 45, 104–112 (1969)
Ragavendra, M.P., Mahadevappa, K.M., Rai, L., Rangappa, K.S.: Mechanistic investigations of oxidation of amino sugars by sodium N-chloro-p-toluenesulfonamide in alkaline medium. J. Carbohydr. Chem. 16, 343–358 (1997)
Shetti, N.P., Hegde, R.N., Nandibewoor, S.T.: Structure reactivity and thermodynamic analysis on the oxidation of amphicillin drug by copper(III) complex in aqueous alkaline medium (stopped flow technique). J. Mol. Struct. 930, 180–186 (2009)
Moelwyn-Hughes, E.A.: Kinetics of Reaction in Solutions, p. 297. Oxford University Press, London (1947)
Krishenbaum, L.J., Mrozowski, L.: Kinetics of silver(III) decomposition in diluted acid. Inorg. Chem. 17, 3718–3719 (1978)
Banerjee, R., Das, K., Das, A., Dasgupta, S.: Kinetics of silver(I)-catalyzed oxidation of formic acid by the (ethylenebis(biguanidine)) silver(III) cation in acid perchlorate media. Inorg. Chem. 28, 585–588 (1989)
Banerjee, R., Das, R., Mukhopadhyay, S.: Kinetics of the silver(I)-catalysed oxidation of H3PO2 with [ethylenebis(biguanide)]silver(III) cation in aqueous perchloric acid solution. J. Chem. Soc. Dalton Trans., 1317–1321 (1992). doi:10.1039/DT9920001317
Crouthamel, C.E., Hayes, A.M., Martin, D.S.: Ionization and hydration equilibria of periodic acid. J. Am. Chem. Soc. 73, 82–87 (1951)
Bailar, J.C., Emeleus, H.J., Nyholm, S.R., Trotman-Dikenson, A.F.: Comprehensive Inorganic Chemistry, vol. 2, p. 1456. Pergamon Press, Oxford (1975)
Bhattacharya, S., Saha, B., Datta, A., Banerjee, P.: Electron transfer reactions of Ni(III) and Ni(IV) complexes. Coord. Chem. Rev. 170, 47–74 (1988)
Haines, R.I., McAuley, A.: Synthesis and reactions of nickel(III) complexes. Coord. Chem. Rev. 39, 77–82 (1981)
Cotton, F.A., Wilkinson, G.: Advanced Inorganic Chemistry, p. 153. Wiley Eastern, New York (1996)
Singh, H.S., Singh, R.K., Singh, S.M., Sisodia, A.K.: A kinetic and mechanistic study of oxidation of tartaric acid by potassium bromate in perchloric acid medium catalysed by Ru(III). J. Phys. Chem. 81, 1044–1047 (1977)
Chimatadar, S.A., Kini, A.K., Nandibewoor, S.T.: Ruthenium(III) catalysed oxidation of l-alanine by alkaline permanganate: A kinetic and mechanistic approach. Inorg. React. Mech. 5, 231–244 (2005)
Desai, S.M., Halligudi, N.N., Nandibewoor, S.T.: Kinetics and mechanism of ruthenium(III)-catalysed oxidation of allyl alcohol by acid bromate-autocatalysis in catalysis. Transition Met. Chem. 27, 207–212 (2002)
Shetter, R.S., Nandibewoor, S.T.: Kinetic, mechanistic and spectral investigations of ruthenium(III)-catalysed oxidation of 4-hydroxycoumarin by alkaline diperiodatonickelate(IV) (stopped flow technique). J. Mol. Catal. 238A, 137143 (2005)
Veeresh, S., Veeresh, T.M., Nandibewoor, S.T.: Ruthenium(III) catalysed oxidation of l-leucine by a new oxidant, diperiodatoargentate(III) in aqueous alkaline medium. Polyhydron 26, 431–436 (2006)
Rangappa, K.S., Raghavendra, M.P., Mahadevappa, D.S., Channegouda, D.: Sodium N-chlorobenzenesulfonamide as a selective oxidant for hexosamines in alkaline medium: A kinetic and mechanistic study. J. Org. Chem. 63, 531–536 (1998)
Kamble, D.L., Nandibewoor, S.T.: Osmium(VIII)/ruthenium(III) catalysis of periodate oxidation of acetaldehyde in aqueous alkaline medium. J. Phys. Org. Chem. 11, 171–176 (1998)
Weissberger, A., Lewis, E.S. (eds.): Investigations of Rates and Mechanism of Reactions in Techniques of Chemistry, vol. 4, p. 421. Wiley, New York (1974)
Martinez, M., Pitarque, M.A., Eldik, R.V.: Outer-sphere redox reactions of [CoIII(NH3)5(HxPyOz)](m−3)− complexes. A temperature- and pressure-dependence kinetic study on the influence of the phosphorus oxoanions. J. Chem. Soc. Dalton Trans. 2665–2671 (1996). doi:10.1039/DT9960002665
Farokhi, S.A., Nandibewoor, S.T.: Kinetic mechanistic and spectral studies for the oxidation of sulfanilic acid by alkaline hexacynoferrate(III). Tetrahedron 59, 7595–7602 (2003)
Acknowledgments
The authors thank the UGC, New Delhi, for the sanction of research grant (F. 36-136/2008 (SR) dated 27-03-2009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
According to Scheme 3:
The total concentration of DPA is given by (where t and f refer to total and free concentrations):
Therefore:
In view of the low [DPA] and [\( {\text{H}}_{3} {\text{IO}}_{6}^{2 - } \)] values used:
Similarly:
and in view of the low Ru(III) concentrations used:
Substituting Eqs. A2–A5 into A1 gives:
The terms (K 3[\( {\text{H}}_{3} {\text{IO}}_{6}^{2 - } \)][d-Glu]) and (K 1 K 3[d-Glu][OH][\( {\text{H}}_{3} {\text{IO}}_{6}^{2 - } \)]) of the denominator are neglected in the view of low concentrations of d-Glu and periodate. Similarly, the rate law for the osmium(VIII) catalyzed reaction was similarly derived.
Rights and permissions
About this article
Cite this article
Sataraddi, S.R., Nandibewoor, S.T. Oxidation of d-Glucose by Silver(III) Periodate Complex in the Presence of Ru(III)/Os(VIII) as a Homogeneous Catalyst: A Comparative Mechanistic Study (Stopped Flow Technique). J Solution Chem 42, 897–915 (2013). https://doi.org/10.1007/s10953-013-0002-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0002-1