Abstract
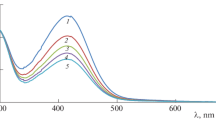
The oxidation of a heterocyclic compound—barbituric acid (BBA) by diperiodatoargentate(III) (DPA) was carried out in the absence and presence of ruthenium(III) catalyst in alkaline medium with a constant ionic strength of 0.20 mol dm–3 at 298 K. The reaction was monitored spectrophotometrically. The reaction was of first order with respect to [DPA] and was less than unity order with respect to [BBA] in both catalyzed and uncatalyzed cases. Positive and negative fractional order in [OH–] for uncatalyzed and Ru(III) catalyzed reaction respectively was observed, whereas perioadate has retarding effect in both the cases. A unity order with respect to [Ru(III)] was observed. The uncatalyzed reaction in alkaline medium has been shown to proceed via a DPA–BBA complex, which decomposes in a rate determining step to give the free radicals, which is followed by other fast steps to give the products. Whereas in catalyzed reaction, it has been shown to proceed via a Ru(III)–BBA complex, and similar other steps as in uncatalyzed reaction to give the products. The reaction constants involved in the various steps involved in the mechanisms were calculated for both the reactions. The catalytic constant (kC) was also calculated for catalyzed reaction at four temperatures. The activation parameters with respect to slow step of the mechanism and also the thermodynamic data for all the equilibrium steps were determined and discussed.









Similar content being viewed by others
REFERENCES
R. H. Garrett and C. M. Grisham, Principals of Biochemistry with a Human Focus (Brooks/Cole Thomson Learning, U. S., 1997).
D. J. Brown, Heterocyclic Compounds: The Pyrimidines (Interscience, New York, 1994), Vol. 52.
E. G. Brown, Ring Nitrogen and Key Biomolecules: The Biochemistry of N-Heterocycles (Kluwer Academic, Boston, 1998).
H. C. Box and E. E. Budzinski, J. Chem. Phys. 59, 1588 (1973).
E. R. Garrett, J. T. Bojarski and G. J. Yakatan, J. Pharm. Sci. 60, 1145 (1971).
W. Löscher and M. A. Rogawski, Epilepsia 53, 12 (2012).
S. B. Konnur and S. T. Nandibewoor, Russ. J. Phys. Chem. A. 93, 1686 (2019)
J. H. Shan, H. Y. Wang, C. Y. Song, and F. Wang, Bull. Chem. Soc. Ethiop. 23, 297 (2009).
R. S. Shettar and S. T. Nandibewoor, J. Mol. Catal. A: Chem. 234, 137 (2005).
B. Sethuram, Some Aspects of Electron-Transfer Reactions Involving Organic Molecules (Allied, New Delhi, 2003), p. 151.
A. Kumar and P. Kumar, J. Phys. Org. Chem. 12, 79 (1999).
C. Yang, Z. Zhang, and J. Wang, Luminiscence 25, 36 (2010).
S. Oi, Y. Ogino, S. Fukita, and Y. Inoue, Org. Lett. 4, 1783 (2002).
L. Ackermann, S. I. Kozhushkov, and D. S. Yufit, Chem. Eur. 18, 12068 (2012).
Z. F. Ke and T. R. Cundari, Organometallics 29, 821 (2010).
C. S. Reddy and T. Vijaykumar, Indian J. Chem. A 34, 615 (1995).
P. A. Magdum, A. M. Bagoji, and S. T. Nandibewoor, J. Phys. Org. Chem. 28, 743 (2015).
G. H. Jeffery, J. Bassett, J. Mendham, and R. C. Denney, Vogel’s Textbook of Quantitative Chemical Analysis, 5th ed. (Longmans Singapore, Singapore, 1996), pp. 467, 391.
S. Kato and G. Dryhurst, J. Electroanal. Chem. 80, 181 (1977).
R. V. Jagdeesh and Puttaswamy, J. Phys. Org. Chem. 21, 844 (2008).
E. A. Moelwyn-Hughes, Kinetics of Reactions in Solutions (Oxford Univ. Press, London, 1947), p. 297.
L. J. Krishenbaum and L. Mrozowski, Inorg. Chem. 17, 3718 (1978).
R. Banerjee, R. Das, and S. Mukhopadhyay, J. Chem. Soc., Dalton Trans. 28, 1317 (1992).
C. E. Crouthamel, A. M. Hayes, and D. S. Martin, J. Am. Chem. Soc. 73, 82 (1951).
P. Jayaprakash Rao, B. Sethuram, and T. Navneeth Rao, React. Kinet. Catal. Lett. 29, 289 (1985).
S. Bhattacharya, B. Saha, A. Datta, and P. Banerjee, Coord. Chem. Rev. 170, 47 (1998).
R. Chang, Physical Chemistry with Applications to Biological Systems (McMillan, New York, 1981), p. 326.
T. S. Kiran and S. T. Nandibewoor, J. Chem. Res. 6, 431 (2006).
K. S. Rangappa, M. P. Raghavendra, D. S. Mahadevappa, and D. Channegouda, J. Org. Chem. 63, 531 (1998).
A. Weissberger and E. S. Lewis, Investigation of Rates and Mechanism of Reactions in Techniques of Chemistry (Wiley Interscience, New York, 1974), Vol. 4, pp. 421.
T. S. Kiran, C. V. Hiremath, and S. T. Nandibewoor, Appl. Catal. A 305, 79 (2006).
R. E. Connick and D. A. Fine, J. Am. Chem. Soc. 82, 4187 (1960).
F. A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann, Advanced Inorganic Chemistry, 6th ed. (Wiley, New York, 1999).
S. Sandu, B. Sethuram, and T. N. Rao, J. Indian Chem. Soc. 60, 198 (1983).
H. P. Panda and B. D. Sahu, Indian J. Chem. 28A, 323 (1989).
V. Tegginamath, C. V. Hiremath, and S. T. Nandibewoor, J. Phys. Org. Chem. 20, 55 (2007).
S. J. Malode, J. C. Abbar, and S. T. Nandibewoor, Inorg. Chim. Acta 363, 2430 (2010).
E. A. Moelwyn-Hughes, Physical Chemistry (Pergamon, New York, 1961).
E. S. Amis, Solvents Effect on Reaction Rates and Mechanisms (Academic, New York, 1966).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bagoji, A.M., Konnur, S.B., Gokavi, N.M. et al. Silver(III) Periodate Complex—An Oxidant for Free Radical Induced Uncatalyzed and Ruthenium(III) Catalyzed Oxidation of Barbituric Acid. Russ. J. Phys. Chem. 94, 2010–2023 (2020). https://doi.org/10.1134/S0036024420100052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420100052