Abstract
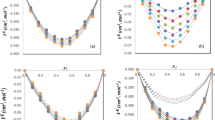
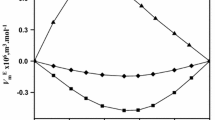
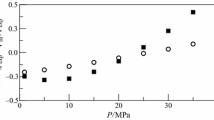
Densities of glycerol (1) + tert-butanol (2) mixtures were measured over the temperature range 293.15 to 348.15 K at atmospheric pressure, over the entire composition range, with a vibrating tube densimeter. Excess molar volumes, apparent and partial molar volumes of glycerol and tert-butanol, thermal isobaric expansivities of the mixture and partial molar expansivities of the components were calculated. The excess molar volumes of the mixtures are negative at all temperatures, and deviations from ideality increase with increasing temperature. Excess molar volumes were fitted to the Redlich–Kister equation. Partial molar volumes of glycerol decrease with increasing tert-butanol concentration. The temperature dependence of the partial molar volumes of glycerol is characterized by an inversion at x 2≈0.7. “Negative expansion” of the limiting partial volumes of glycerol was observed.








Similar content being viewed by others
References
Towey, J.J., Soper, A.K., Dougan, L.: The structure of glycerol in the liquid state: a neutron diffraction study. Phys. Chem. Chem. Phys. 13, 9397–9406 (2011)
Champeney, D.C., Joarder, R.N., Dore, J.C.: Structural studies of liquid D-glycerol by neutron-diffraction. Mol. Phys. 58, 337–347 (1986)
Chelli, R., Gervasio, F.L., Gellini, C., Procacci, P., Cardini, G., Schettino, V.: Density functional calculation of structural and vibrational properties of glycerol. J. Phys. Chem. A 104, 5351–5357 (2000)
Chelli, R., Procacci, P., Cardini, G., Della Valle, R.G., Califano, S.: Glycerol condensed phases Part I. A molecular dynamics study. Phys. Chem. Chem. Phys. 1, 871–877 (1999)
van Koningsveld, H.: The crystal structure of glycerol and its conformation. Recl. Trav. Chim. Pays-Bas. 87, 243–254 (1968)
Dawidowski, J., Bermejo, F.J., Fayos, R., Perea, R.F., Bennington, S.M., Criado, A.: Coherent neutron scattering response from glassy glycerol. Phys. Rev. E 53, 5079–5088 (1996)
Garawia, M., Dorea, J.C., Champeney, D.C.: Structural studies of liquid D-glycerol II. Molecular conformation and long range correlations. Mol. Phys. 62, 475–487 (1987)
Dashnau, J.L., Nucci, N.V., Sharp, K.A., Vanderkooi, J.M.: Hydrogen bonding and the cryoprotective properties glycerol/water mixtures. J. Phys. Chem. B 110, 13670–13677 (2006)
Jain, P., Levchenko, A., Yu, P., Sen, S.: Molecular dynamics in supercooled glycerol: results from 13C NMR spectroscopy. J. Chem. Phys. 130, 194506–194506-5 (2009)
Marcus, Y.: Some thermodynamic and structural aspects of mixtures of glycerol with water. Phys. Chem. Chem. Phys. 2, 4891–4896 (2000)
Westh, P., Rasmussen, E.L., Koga, Y.: Intermolecular Interactions in ternary glycerol–sample–H2O: towards understanding the Hofmeister series (V). J. Solution Chem. 40, 93–105 (2011)
Zelent, B., Nucci, N.V., Vanderkooi, J.M.: Liquid and ice water and glycerol/water glasses compared by infrared spectroscopy from 295 to 12 K. J. Phys. Chem. A 108, 11141–11150 (2004)
Callam, C.S., Singer, S.J., Lowary, T.L., Hadad, C.M.: Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous phases: effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems. J. Am. Chem. Soc. 123, 11743–11754 (2001)
Chelli, R., Procacci, P., Cardini, G., Califano, S.: Glycerol condensed phases. Part II: a molecular dynamics study of the conformational structure and hydrogen bonding. Phys. Chem. Chem. Phys. 1, 879–885 (1999)
Perron, G., Desnoyers, J.E.: Heat capacities and volumes of interaction between mixtures of alcohols in water at 298.15 K. J. Chem. Thermodyn. 13, 1105–1121 (1981)
Alary, I.F., Simard, M.A., Dumont, J., Jolicoeur, C.: Simultaneous flow measurement of specific heats and thermal expansion coefficients of liquids: aqueous t-BuOH mixtures and neat alkanols and alkanediols at 25 °C. J. Solution Chem. 11, 755–776 (1982)
Tamura, K., Osaki, A., Koga, Y.: Compressibilities of aqueous tert-butanol in the water-rich region at 25 °C: partial molar fluctuations and mixing schemes. Phys. Chem. Chem. Phys. 1, 121–126 (1999)
Franks, F., Smith, H.T.: Precision densities of dilute aqueous solutions of the isomeric butanols. J. Chem. Eng. Data 13, 538–541 (1968)
Sakurai, M., Nakamura, K., Nitta, K.: Volumetric properties of dilute aqueous alcohol solutions at different temperatures. Bull. Chem. Soc. Jpn. 67, 1580–1587 (1994)
Sakurai, M.: Partial molar volumes in aqueous mixtures of nonelectrolytes. I. t-Butyl alcohol. Bull. Chem. Soc. Jpn. 60, 1–7 (1987)
Hvidt, A., Moss, R., Nielsen, G.: Volume properties of aqueous solutions of tert-butyl alcohol at temperatures between 5 and 25 °C. Acta Chem. Scand. 32, 274–280 (1978)
Kim, E.S., Marsh, K.N.: Excess volumes for 2-methyl-2-propanol–water at 5 K intervals from 303.15 to 323.15 K. J. Chem. Eng. Data 33, 288–292 (1988)
Egorov, G.I., Makarov, D.M.: Densities and volume properties of (water + tert-butanol) over the temperature range of (274.15 to 348.15) K at pressure of 0.1 MPa. J. Chem. Thermodyn. 43, 430–441 (2011)
Rabinovich, V.A., Havin, Z.Ya.: In: Potekhin, A.A., Efimov, A.I. (eds.) Short Chemical Handbook, 3th edn. Khimiya Press, Moscow (1991)
Lide, D.R. (ed.): Handbook of Chemistry and Physics, 82nd edn. CRC Press, New York (2001)
Weissberger, F., Proskauer, E.S., Riddik, J.A., Toops, E.E.: Organic Solvents. Physical Properties and Methods of Purification. Interscience, New York (1955)
Egorov, G.I., Syrbu, A.A., Kolker, A.M.: Volume properties of the H2O–DMF mixture at the pressure 0.101 MPa in the temperature range 278.15–323.15 K. Russ. J. Gen. Chem. 72, 693–696 (2002)
Egorov, G.I., Afanas’ev, V.N., Kolker, A.M.: VTx properties of the system water–2-propanol in the range 275.15–338.15 K. Russ. J. Gen. Chem. 74, 171–173 (2004)
Egorov, G.I., Makarov, D.M.: The bulk properties of ethylene glycol–dimethylsulfoxide mixtures over the temperature range 278–323 K at p=0.1 MPa. Russ. J. Phys. Chem. A 82, 1778–1784 (2008)
Egorov, G.I., Makarov, D.M.: The bulk properties of the water–dimethylsulfoxide system at 278–323.15 K and atmospheric pressure. Russ. J. Phys. Chem. A 83, 693–698 (2009)
Egorov, G.I., Makarov, D.M., Kolker, A.M.: Volumetric properties of the water–ethylene glycol mixtures in the temperature range 278–333.15 K at atmospheric pressure. Russ. J. Gen. Chem. 80, 1577–1585 (2010)
Egorov, G.I., Gruznov, E.L., Kolker, A.M.: p–V m –T–x properties of water–acetone mixtures over the temperature range 298–323 K and pressures from 1 to 1000 bar: isothermal compressibility, volume expansion coefficients and inner pressure of water–acetone mixtures. Russ. J. Phys. Chem. A 70, 197–204 (1996)
Egorov, G.I., Syrbu, A.A., Kolker, A.M.: The p–V m –x properties of water–acetamide mixtures at 298.15 K over the pressure range of 1–1000 bar. J. Phys. Chem. A 73, 1949–1951 (1999)
Egorov, G.I., Kolker, A.M.: Effect of pressure and temperature on volume properties of water–N,N-dimethylformamide mixtures. J. Mol. Liq. 106, 239–248 (2003)
Egorov, G.I., Makarov, D.M.: Compressibility coefficients of water–2-propanol mixtures over the temperature and pressure ranges 278–323.15 K and 1–1000 bar. Russ. J. Phys. Chem. A 82, 1037–1041 (2008)
Egorov, G.I., Makarov, D.M.: The compressibility of water–dimethyl sulfoxide mixtures over the temperature and pressure ranges 278–323.15 K and 1–1000 bar. Russ. J. Phys. Chem. A 83, 2058–2065 (2009)
Egorov, G.I., Makarov, D.M., Kolker, A.M.: Densities and volumetric properties of ethylene glycol + dimethylsulfoxide mixtures at temperatures of (278.15 to 323.15) K and pressures of (0.1 to 100) MPa. J. Chem. Eng. Data 55, 3481–3488 (2010)
Gordon, A.J., Ford, R.A.: The Chemist’s Companion. A Handbook of Practical Data, Techniques, and References. Wiley, New York (1972)
Xu, L., Hu, X., Lin, R.: Volumetric properties of glycerol with N,N-dimethylformamide and with water at 25 and 35 °C. J. Solution Chem. 32, 363–370 (2003)
Li, Q.-S., Su, M.-G., Wang, S.: Densities and excess molar volumes for binary glycerol + 1-propanol, + 2-propanol, + 1,2-propanediol, and + 1,3-propanediol mixtures at different temperatures. J. Chem. Eng. Data 52, 1141–1145 (2007)
Ge, M.-L., Ma, J.-L., Chu, B.: Densities and viscosities of propane-1,2,3-triol + ethane-1,2-diol at T=(298.15 to 338.15) K. J. Chem. Eng. Data 55, 2649–2651 (2010)
Martınez, S., Garriga, R., Perez, P., Gracia, M.: Densities and viscosities of binary mixtures of butanenitrile with butanol isomers at several temperatures. J. Chem. Eng. Data 45, 1182–1188 (2000)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Torres, R.B., Marchiore, A.C.M., Volpe, P.L.O.: Volumetric properties of binary mixtures of (water + organic solvents) at temperatures between T=288.15 K and T=303.15 K at p=0.1 MPa. J. Chem. Thermodyn. 38, 526–541 (2006)
Narten, A.H., Sandler, S.I.: X-ray diffraction study of liquid tertiary butyl alcohol at 26 °C. J. Chem. Phys. 71, 2069–2073 (1979)
Bowron, D.T., Finney, J.L., Soper, A.K.: The structure of pure tertiary butanol. Mol. Phys. 93, 531–543 (1998)
Kusalik, P.G., Lyubartsev, A.P., Bergman, D.L., Laaksonen, A.: Computer simulation study of tert-butyl alcohol. 2. Structure in aqueous solution. J. Phys. Chem. B 104, 9533–9539 (2000)
Fukasawa, T., Tominaga, Y., Wakisaka, A.: Molecular association in binary mixtures of tert-butyl alcohol–water and tetrahydrofuran–heavy water studied by mass spectrometry of clusters from liquid droplets. J. Phys. Chem. A 108, 59–63 (2004)
Wojtkow, D., Czarnecki, M.A.: Effect of temperature and concentration on the structure of tert-butyl alcohol/water mixtures: near-infrared spectroscopic study. J. Phys. Chem. A 109, 8218–8224 (2005)
Yoshida, K., Yamaguchi, T., Kovalenko, A., Hirata, F.: Structure of tert-butyl alcohol–water mixtures studied by the RISM theory. J. Phys. Chem. B 106, 5042–5049 (2002)
Nath, P.P., Sarkar, S., Krishna, P.S.R., Joarder, R.N.: Intermolecular structure of liquid D-tert-butanol by neutron-diffraction data. Appl. Phys. A 74, S348–S351 (2002)
Hamilton, D., Stokes, R.H.: Apparent molar volumes of urea in several solvents as functions of temperature and concentration. J. Solution Chem. 1, 213–221 (1972)
Abrosimov, V.K., Ivanov, E.V.: Water, structure, state and solvation, achievements of last years. In: Kutepov, A.M. (ed.) Water in Nonaqueous Solutions: State and Solvation, pp. 277–346. Nauka, Moscow (2003)
Nakajima, T., Komatsu, T., Nakagawa, T.: Apparent molal volumes and adiabatic compressibilities of n-alkanols and α,ω-alkane diols in dilute aqueous solutions at 5, 25, and 45 °C. I. Apparent molal volumes. Bull. Chem. Soc. Jpn. 48, 783–787 (1975)
Sakurai, M.: Partial molar volumes in aqueous mixtures of nonelectrolytes. II. Isopropyl alcohol. J. Solution Chem. 17, 267–276 (1988)
Franks, F., Smith, H.T.: Volumetric properties of alcohols in dilute aqueous solutions. Trans. Faraday Soc. 64, 2962–2972 (1968)
de Visser, C., Perron, G., Desnoyers, J.E.: The heat capacities, volumes, and expansibilities of tert-butyl alcohol–water mixtures from 6 to 65 °C. Can. J. Chem. 55, 856–862 (1977)
Hyncica, P., Hnedkovsky, L., Cibulka, I.: Partial molar volumes of organic solutes in water. XIII. Butanols (aq) at temperatures T=298 K to 573 K and at pressures up to 30 MPa. J. Chem. Thermodyn. 38, 418–426 (2006)
Sakurai, M., Nakagawa, T.: Densities of dilute solutions of water in n-alkanols at 278.15, 288.15, 298.15, 308.15, and 318.15 K. Partial molar volumes of water in n-alkanols. J. Chem. Thermodyn. 16, 171–174 (1984)
Sakurai, M.: Partial molar volumes in aqueous mixtures of nonelectrolytes. III. t-Pentyl alcohol. J. Solution Chem. 18, 37–44 (1989)
Egorov, G.I., Makarov, D.M.: Volumetric properties of the binary mixture of ethylene glycol + tert-butanol at T=(278.15,288.15,298.15,308.15,323.15,333.15,348.15) K under atmospheric pressure. J. Mol. Liq. (in press)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents: Physical Properties and Methods of Purification; Techniques of Chemistry. Wiley-Interscience, New York (1986)
Soujanya, J., Satyavathi, B., Vittal Prasad, T.E.: Experimental (vapour + liquid) equilibrium data of (methanol + water), (water + glycerol) and (methanol + glycerol) systems at atmospheric and sub-atmospheric pressures. J. Chem. Thermodyn. 42, 621–624 (2010)
Adamenko, I.I., Bulavin, L.A., Ilyin, V., Zelinsky, S.A., Moroz, K.O.: Anomalous behavior of glycerol–water solutions. J. Mol. Liq. 127, 90–92 (2006)
Sanz, M.T., Blanco, B., Beltran, S., Cabezas, J.L., Coca, J.: Vapor liquid equilibria of binary and ternary systems with water, 1,3-propanediol, and glycerol. J. Chem. Eng. Data 46, 635–639 (2001)
Verhoeye, L., Lauwers, E.: Vapor–liquid equilibrium of the system 2-propanol–water–1,2,3-propanetriol at 760 mm of Hg. J. Chem. Eng. Data 14, 306–309 (1969)
Sadek, H., Habez, A.M., Khalil, F.X.: Conductance of KIO3 in glycerol–water mixtures. Electrochim. Acta 14, 1089–1096 (1969)
Murthy, M.N., Subrahmahyan, S.V.: Behaviour of excess heat capacity of aqueous non-electrolytes. Indian J. Pure Appl. Phys. 15, 485–489 (1977)
Uosaki, Y., Kitaura, S., Moriyoshi, T.: Static relative permittivities of water + ethane-1,2-diol and water + propane-1,2,3-triol under pressures up to 300 MPa at 298.15 K. J. Chem. Eng. Data 51, 423–429 (2006)
Darbari, G.S., Singh, R.P., Verma, G.S., Rajagopalan, S.: Acoustic absorption in mixtures of glycerol and water below 1 MHz. II. Nuovo Cimento B 52, 1–17 (1967)
Nain, A.K.: Densities and volumetric properties of binary mixtures of aniline with 1-propanol, 2-propanol, 2-methyl-1-propanol, and 2-methyl-2-propanol at temperatures from 293.15 to 318.15 K. Int. J. Thermophys. 28, 1228–1244 (2007)
Anson, A., Garriga, R., Martinez, S., Perez, P., Gracia, M.: Densities and viscosities of binary mixtures of 1-chlorobutane with butanol isomers at several temperatures. J. Chem. Eng. Data 50, 677–682 (2005)
Kenttaemaa, J., Tommila, E., Martti, M.: Some thermodynamic properties of the system t-butanol + water. Ann. Acad. Sci. Fenn. Ser. A2 93, 1–20 (1959)
TRC Thermodynamic Tables. Non-Hydrocarbons. Thermodynamic Research Center, Texas A&M University, College Station, TX, d-5030 (1966)
Langa, E., Mainar, A.M., Pardo, J.I., Urieta, J.S.: Excess enthalpy, density, and speed of sound for the mixtures β-pinene + 2-methyl-1-propanol or 2-methyl-2-propanol at several temperatures. J. Chem. Eng. Data 52, 2182–2187 (2007)
Kubota, H., Tanaka, Y., Makita, T.: Volumetric behavior of pure alcohols and their water mixtures under high pressure. Inter. J. Thermophys. 8, 47–70 (1987)
Harris, K.R., Newitt, P.J., Back, P.J., Woolf, L.A.: Thermodynamic property measurements for 2-methyl-2-propanol + water from the freezing surface to 75 °C. High Temp., High Press. 30, 51–62 (1998)
Acknowledgement
This work was supported by the Russian Foundation for Basic Research (project 09-03-97501a).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egorov, G.I., Makarov, D.M. Volumetric Properties of Binary Mixtures of Glycerol + tert-Butanol over the Temperature Range 293.15 to 348.15 K at Atmospheric Pressure. J Solution Chem 41, 536–554 (2012). https://doi.org/10.1007/s10953-012-9813-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9813-8