Abstract
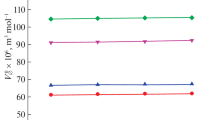
The partial molar volume at infinite dilution of aqueous solutions of 3-aminopropanoic acid, 4-aminobutanoic acid, 5-aminopentanoic acid and 6-aminohexanoic acid were determined at T=(293.15,298.15,303.15 and 308.15 K) from density measurements. The thermodynamic behavior of the aqueous α,ω-amino acid solutions is compared with that reported for α-amino acids in water. The interaction volume is calculated and the influence of charged and uncharged groups of the amino acids is discussed in terms of solute–solvent interactions.
Similar content being viewed by others
References
Andrews, J.I., Tabor, A.B.: Forming stable helical peptides using natural and artificial amino acids. Tetrahedron 55, 11711–11743 (1999)
Soto, A., Arce, A., Khoshkbarchib, M.K., Vera, J.H.: Effect of the cation and the anion of an electrolyte on the solubility of DL-aminobutyric acid in aqueous solutions: measurement and modeling. Biophys. Chem. 73, 77–83 (1998)
Lu, Y., Xie, W., Lu, J.: The enthalpic interaction parameters of α-aminobutyric acid and alkali metal halides in water at 298.15 K. Thermochim. Acta 385, 1–4 (2002)
Kharakoz, D.P.: Volumetric properties of proteins and their analogs in diluted water solutions. 1. Partial volumes of amino acids at 15–55 °C. Biophys. Chem. 34, 115–125 (1989)
Millero, F.J., Lo Surdo, A., Shin, C.: The apparent molal volumes and adiabatic compressibilities of aqueous amino acids at 25 °C. J. Phys. Chem. 82, 784–792 (1978)
Mishra, A.K., Ahluwalia, J.C.: Apparent molal volumes of amino acids, N-acetylamino acids, and peptides in aqueous solutions. J. Phys. Chem. 88, 86–92 (1984)
Chalikian, T.V., Kharakoz, D.P., Sarvazyan, A.P.: Ultrasonic study of proton-transfer reactions in aqueous solutions of amino acids. J. Phys. Chem. 96, 876–883 (1992)
Abrosimov, B.K., Sibrina, G.K.: Volume properties of α-amino acids in limiting dilute aqueous solutions at different temperatures. Russ. Chem. Bull. 38, 1143–1146 (1989)
Romero, C.M., Negrete, F.: Effect of temperature on partial molar volumes and viscosities of aqueous solutions of α-DL-aminobutyric acid, DL-norvaline and DL-norleucine. Phys. Chem. Liq. 42, 261–267 (2004)
Yan, Z., Wang, J., Liu, W., Lu, J.: Apparent molar volumes and viscosity B-coefficients of some α-amino acids in aqueous solutions from 278.15 to 308.15 K. Thermochim. Acta 334, 17–27 (1999)
Chalikian, T.V., Sarvazyan, A.P., Breslauer, K.J.: Partial molar volumes, expansibilities, and compressibilities of α,ω-aminocarboxylic acids in aqueous solutions between 18 and 55 °C. J. Phys. Chem. 97, 13017–13026 (1993)
Likhodi, O., Chalikian, T.V.: Differential hydration of α,ω-aminocarboxylic acids in D2O and H2O. J. Am. Chem. Soc. 122, 7860–7868 (2000)
Shahidi, F., Farell, P.G.: Partial molar volumes of organic compounds in water. Part 4. Aminocarboxylic acids. J. Chem. Soc., Faraday Trans. 1 74, 858–868 (1978)
Badelin, V.G., Tyunina, E.Y.: Thermodynamic characteristics of solution and viscous flow of amino acids in water at 298.15 K. Russ. J. Gen. Chem. 78, 1155–1160 (2008)
Hakin, A.W., Liu, J.L.: The calorimetric and volumetric properties of selected α-amino acids and α,ω-amino acids in water at T=(288.15,298.15,313.15, and 328.15) K and p=0.1 MPa. J. Solution Chem. 35, 1157–1171 (2006)
Ahluwalia, J.C., Ostiguy, C., Perron, G., Desnoyers, J.E.: Volumes and heat capacities of some amino acids in water at 25 °C. Can. J. Chem. 55, 3364–3367 (1977)
Banipal, T.S., Kapoor, P.: Partial molal volumes and expansibilities of some amino acids in aqueous solutions. J. Indian Chem. Soc. 76, 431–437 (1999)
Wadi, R.K., Islam, M.N., Goyal, R.K.: Equilibrium and transport properties of amino acid solutions: Part 1. Temperature dependence of apparent molar volume in aqueous solutions between 288 and 308 K. Indian J. Chem., Sect A 29, 1055–1059 (1990)
Cabani, S., Conti, G., Matteoli, E.: Volumetric properties of amphionic molecules in water. Part 1. Volume changes in the formation of zwitterionic structures. J. Chem. Soc., Faraday Trans. 1 77, 2377–2384 (1981)
Cabani, S., Conti, G., Matteoli, E.: Volumetric properties of amphionic molecules in water. Part 2. Thermal expansibility and compressibility related to the formation of zwitterionic structures. J. Chem. Soc., Faraday Trans. 1 77, 2385–2394 (1981)
Devine, W., Lowe, B.: Viscosity B-coefficients at 15 and 25 °C for glycine, β-alanine, 4-amino-n-butyric acid, and 6-amino-n-hexanoic acid in aqueous solution. J. Chem. Soc. A 2113–2116 (1971)
Taulier, N., Chalikian, T.V.: Volumetric effects of ionization of amino and carboxyl termini of α,ω-aminocarboxylic acids. Biophys. Chem. 104, 21–36 (2003)
Lepori, L., Mollica, V.: Volume changes in the proton ionization of α,ω-aminocarboxylic acids in aqueous solution. Z. Phys. Chem. N. F. 123, 51–66 (1980)
Ramasami, P., Kakkar, R.: Partial molar volumes and adiabatic compressibilities at infinite dilution of aminocarboxylic acids and glycylglycine in water and aqueous solutions of sodium sulphate at (288.15, 298.15 and 308.15) K. J. Chem. Thermodyn. 38, 1385–1395 (2006)
Wadi, R.K., Goyal, R.K.: Temperature dependence of apparent molar volumes and viscosity B-coefficients of amino acids in aqueous potassium thiocyanate solutions from 15 to 35 °C. J. Solution Chem. 21, 163–170 (1992)
Natarajan, M., Wadi, R.K., Gaur, H.C.: Apparent molar volumes and viscosities of some α- and α,ω-amino acids in aqueous ammonium chloride solutions at 298.15 K. J. Chem. Eng. Data 35, 87–93 (1990)
Islam, M.N., Wadi, R.K.: Temperature dependence of apparent molar volumes and viscosity B-coefficients of amino acids in aqueous sodium sulfate solutions from 15 to 35 °C. Phys. Chem. Liq. 41, 533–544 (2003)
Weissberger, A.: Techniques in Chemistry. Vol. I, Part 4: Methods of Chemistry. Wiley, New York (1972)
Ramasami, P., Kakkar, R.: Partial molar volumes and adiabatic compressibilities at infinite dilution of aminocarboxylic acids and glycylglycine in water and aqueous solutions of sodium sulphate at (288.15, 298.15 and 308.15) K. J. Chem. Thermodyn. 38, 1385–1395 (2006)
Daniel, J., Cohn, E.J.: Studies in the physical chemistry of amino acids, peptides and related substances: VI. The densities and viscosities of aqueous solutions of amino acids. J. Am. Chem. Soc. 58, 415–423 (1936)
Gucker, Jr.F.T., Allen, T.W.: The densities and specific heats of aqueous solutions of DL-α-alanine, β-alanine and lactamide. J. Am. Chem. Soc. 64, 191–199 (1942)
Romero, C.M., Munar, L.R.: Apparent molar volumes of amino acids in very dilute aqueous solutions at 25.00 °C. Phys. Chem. Liq. 36, 83–90 (1998)
Bhattacharya, M.M., Sengupta, M.: Ion–solvent interaction of aminoacids: IV. Apparent molal volumes of amino acids in neutral, acidic and alkaline media at different temperatures. J. Indian Chem. Soc. 62, 959–964 (1985)
Gopal, R., Agarwal, D.K., Kumar, R.: Variation of limiting apparent molal volume with temperature in some amino acids in aqueous solutions. Indian J. Chem. 11, 1061–1062 (1973)
Zhao, H.: Review: Viscosity B-coefficients and standard partial molar volumes of amino acids, and their roles in interpreting the protein (enzyme) stabilization. Biophys. Chem. 122, 157–183 (2006)
Hakin, A.W., Duke, M.M., Groft, L.L., Marty, J.L., Rushfeldt, M.L.: Calorimetric investigations of aqueous amino acid and dipeptide systems from 288.15 to 328.15 K. Can. J. Chem. 73, 725–734 (1995)
Hakin, A.W., Copeland, A.K., Liu, J.L., Marriott, R.A., Preuss, K.E.: Densities, apparent molar volumes, and apparent molar heat capacities of L-arginine, L-proline and D,L-methionine in water at 288.15, 298.15, 313.15, and 328.15 K. J. Chem. Eng. Data 42, 84–89 (1997)
Pierotti, R.A.: A scaled particle theory of aqueous and nonaqueous solutions. Chem. Rev. 76, 717–726 (1976)
Terasawa, S., Itsukl, H., Arakawa, S.: Contribution of hydrogen bonds to the partial molar volumes of nonionic solutes in water. J. Phys. Chem. 79, 2345–2351 (1975)
Edward, J.T., Farrell, P.G.: Relation between van der Waals and partial molal volumes of organic molecules in water. Can. J. Chem. 53, 2965–2970 (1975)
Bondi, A.: van der Waals volumes and radii. J. Phys. Chem. 68, 441–451 (1964)
Pierotti, R.A.: Aqueous solutions of nonpolar gases. J. Phys. Chem. 69, 281–288 (1965)
Lide, D.R. (ed.): CRC Handbook of Chemistry and Physics, 89th edn. CRC Press/Taylor & Francis, Boca Raton (2009)
Kitano, I., Takaha, K., Gemmei-Ide, M.: Raman spectroscopic study on the structure of water in aqueous solution of α,ω-amino acids. J. Colloid Interface Sci. 283, 452–458 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, C.M., Cadena, J.C. Effect of Temperature on the Volumetric Properties of α,ω-Amino Acids in Dilute Aqueous Solutions. J Solution Chem 39, 1474–1483 (2010). https://doi.org/10.1007/s10953-010-9602-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-010-9602-1