Abstract
The sample of nominal composition Bi1.6Pb0.4Sr2Ca2Cu3O10+δ was prepared by the solid-state reaction method and systematically studied for their magnetic properties. The phase identification/gross structural characteristics of synthesized (HTSC) materials explored through powder X-ray diffractometer reveals that all the samples crystallize in orthorhombic structure with lattice parameters a=5.4054 Å, b=5.4111 Å and c=37.0642 Å. The critical transition temperature (T c ) measured by the standard four-probe method has been found to decrease from 108 K. The surface morphology investigated through scanning electron microscope and atomic force microscopy (SEM and AFM) results that voids and grains size increases of the Bi-2223 sample. The structural/microstructural characteristics (TEM) of Bi-based cuprate HTSC phase exhibit curious structural features including the occurrence of super lattice modulation along the b direction and c direction that have been observed. The presence of this streak reveals the presence of planar disorder representing faults in the stacking of CuO2 planes. The variation of magnetization with temperature (M–T curve) of the same Bi-based HTSC materials was measured by cooling the sample in zero fields (ZFC) and an applied field of 0.05T (here after referred as FC).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bi-based ceramic superconductors with T c =110 K may have important implications in various applications. The interest is mainly due to the great potential of this material for use in superconductor devices operated at liquid nitrogen temperatures. Bi2Sr2Ca n Cu n+1O y ceramics (n=0, 1 and 2 with 10, 85, 110 K transition temperatures, respectively) commonly have a multiphase structure. The most important problem in these systems is the difficulty of obtaining phase-pure high T c (n=2, 2223) materials.
A tremendous amount of work on the Bi-2223 system has been carried out since its discovery [1]. In recent investigations on the high-T c superconducting ceramics, the BSCCO system has received much attention because of its transition temperature and its high critical current density. Chemical doping, substitution, addition and diffusion with different elements at various amounts and adjusting the preparation condition play a very important role in high-T c superconductors [2]. It is well known that there are several phases in the BSCCO system. The composition of each phase is expressed by the general formula of (Bi, Pb)2Sr2Ca n−1Cu n O x (n=1, 2, 3) and T c of ∼20, 95 and 110 K, respectively. The phases are called Bi-2201, Bi-2212 and Bi-2223 for n=1, 2, 3, respectively [3]. In these series, Bi-2223 is the most attractive because its superconducting transition temperature, T c , is the highest one, about 110 K. The system Bi–Sr–Ca–Cu–O contains non-toxic elements and its preparation is cheap, which makes it a very promising material for technical applications; however, it is rather difficult to obtain it in an isolated form since the Bi-2212 phase grows prior to the Bi-2223 phase during synthesis [4]. Despite extensive studies, the mechanism of the high-T c superconductivity remains unclear. Dopant may play an important role in the properties of high-temperature superconducting materials. The influence of dopant materials like Al, La, Sb, and Pb on the Bi-based superconductors has been reported by various authors [5].
Many substitutions and dopings have also been used to improve formation and stability of the Bi-2223 phase. Lead (Pb) is the most important doping element that influences the microstructure, phase composition and the related superconducting properties of the BSCCO system. The presence of Pb in the initial mixture, usually as PbO, favors the reaction kinetics of the 110 K (Bi-2223) phase. The Pb addition results in the creation of a superconductivity solid solution Bi-2223 by partial substitution of bismuth (Bi) [5–7].
Superconducting oxides are usually prepared by a solid-state reaction method which utilizes metal oxides and carbonates [8]. It has several disadvantages; inhomogenity, nonuniformity of particle size and shape, high impurity content, lack of reproducibility, high porosity of the sintered samples, and necessity of repeated processing and requirement of higher temperature for long period of sintering. Moreover, residual carbon decreases the critical current density, which is a key parameter for application of superconductor and also enhances the formation of the non-superconducting phases [9]. Several solution methods, such as the sol-gel method [10, 11], the citrate process [12, 13] and spray pyrolysis have been developed to overcome these problems. However, it was almost impossible to obtain gels from the solution of bismuth salt and thus the high-T c phase due to its poor solubility and large difference in the solubility of the metal salts.
In this study, we have investigated the effects of Pb substitution in Bi sites in Bi-2223 superconductors. We have discussed the results of electrical-resistivity measurement (R-T), X-ray diffraction analysis (XRD), Scanning electron microscopy (SEM), Atomic force microscopy (AFM), Transmission electron microscopy (TEM) and magnetic properties measurement.
2 Experimental Details
Bi1.6Pb0.4Sr2Ca2Cu3O10+δ (Bi, Pb)-2223 samples were prepared by a conventional solid-state reaction method. In brief, the powders of high grade purity Bi2O3 (99.99%, Alfa Aesar), PbO (99.99%, Alfa Aesar), SrCO3 (99.9%, Alfa Aesar), CaCO3 (99.9%, Alfa Aesar) and CuO (99.99%, Alfa Aesar) were mixed and ground in an agate mortar pestle, put in cylindrical boats and calcined at 810 ∘C for 24 hrs in air. After that it was mixed and pressed into pellets. Finally, the pellets (samples) were sintered at 848 ∘C for 34 hrs and slowly furnace cooled to room temperature. The homogeneous powder thus formed was converted into the form of pellets before sintering. For this we employed the most widely used technique i.e. dry pressing, which consists of filling a die with powder and pressing at 400 Kg/cm2 into a circular shape. The nominal composition Bi-2223 samples were sintered in air very near to melting point (∼848 ∘C) for 34 hrs and then cooled slowly down to room temperature (∼30 ∘C). The heat treatment schedule for Bi-2223 pellets in programmable temperature controlled silicon carbide (SiC) furnace is shown in Fig. 1.
3 Results and Discussions
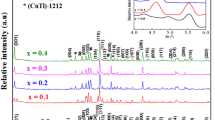
Figure 2 is representative XRD patterns of Bi-2223 after heat treatment at 840∘C for 24 hrs. The diffractograms were recorded with scan speed 2 ∘/minute (0.03∘/s) and in range 15–65∘. The recorded peaks were analyzed and indexed with standard data of ‘Joint Committee on Powder Diffraction Standards/File’ (JCPDS/F). The analysis of diffractograms revealed that the crystal system of the as-synthesized material corresponds to the orthorhombic phase. Lattice parameters determined from the XRD peaks of the high angle range (2θ=20–65∘) were a=5.4054 Å, b=5.4111 Å and c=37.0642 Å for Bi-2223.
The surface morphology and corresponding EDAX pattern in same regions are shown in Figs. 3 (a and b) at a typical operating voltage of 20 kV with resolution limit of 3.5 nm. The surface morphology i.e. pores, voids, size of grains, their orientation etc. are varying, however, the resulting average cationic compositions of pellets were found in 2:2:2:3 ratio in almost each region. The SEM micrograph of Bi-2223 in same region, yields a highly uniform microstructure, randomly oriented grains (crystallites) and small porosity. In this region, the porosity decreases i.e. the connection of grains was more compact in Bi-2223 sample.
The two-dimensional AFM image appears as a nanosphere like structure on the surface as shown in Fig. 4(a). The most pronounced difference in the two layers of oxide is around 37.673 nm. It is noted that fluctuation in the scan profile in the upper layer as well as in the lower layer is due to changes from one grain to another grain of the oxides. Figure 5(a) shows the three-dimensional (3D) view of the same surface. The formation of the humps in some places could be clearly seen, which is due to formation of an oxide layer with different thickness depending on the chemical composition of phases. Figure 5(b) corresponds to the three-dimensional phases and the other two matrices and line traces were used to determine the difference between superconducting layers i.e. the radius profile curve.
The temperature dependence of electrical resistance for Bi-2223 is shown in Fig. 6(a). The transition temperature (T c ) was found ∼108 K for Bi-2223. This is possibly due to the fact that singular Bi-2223 phase could not be synthesized in the present investigation. The phase control in Bi-based superconductors is complicated in comparison to other cuprates. It has been found that when a Bi-2223 phase is annealed in the presence of oxygen, the amount of Bi-2223 compound decreases with time. As discussed above, the impurity phases have been increased during the formation of Bi-2223 phase, which enhanced the transition width (ΔT c ) in this phase. The synthesis process, the sintering time and temperature influence the transition temperature and transition width of the superconducting material and the formation of a single-phase material. For the best samples of Bi-2223, the transition temperature (T c ) was also measured under varying magnetic field (zero to 8 tesla) in the cooling and warming conditions. The variations transition temperatures of the above samples with magnetic fields are shown in Fig. 6(b). It may be inferred from Fig. 6(b) that the transition temperature decreases as the magnetic fields increases.
Some of the typical transmission electron micrographs, corresponding selected area electron diffraction (SAED) patterns and the high-resolution electron microscopic image (lattice fringes) of some local regions of Bi-2223 samples are shown in Fig. 7(a) and (b). The indexing of SAED reveals clearly the existence of Bi-2223 phases. An important microstructural/structural characteristic found in all of the double and triple CuO2 layer Bi-bearing HTSC compounds is the presence of weak super lattice reflections in the selected area electron diffraction patterns. One of the representative [100] selected area electron diffraction patterns from crystallites of Bi-2223 showing the super lattice reflections is given in Fig. 7(a) and (b). It can be seen that in addition to the spots along the c ∗ axis bringing out the presence of Bi-2223 HTSC phase, the diffraction spots along b ∗ (a ∗) are also present. These spots unravel the presence of lattice modulation along the b-direction.
Figure 8 shows the variation of magnetization with temperature (M–T curves) of Bi-based HTSC materials measured by cooling the sample in zero fields (ZFC) and applied field of 0.05 T) (here after referred to as FC). The transition temperature ‘T c ’ and the melting point ‘T m ’ of the Abrikosov-vortex lattice (AVL) are indicated by an arrow. The AVL melting point ‘T m ’ of the same sample was found to be field independent as shown in Fig. 8. As expected, the Bi-2223 sample exhibits diamagnetic behavior. Figure 8 shows the zero-field-cooled (ZFC) and field-cooled (FC) curves of the Bi-2223 sample after final sintering at 848 ∘C. One can notice an increase of T c up to 102 K, which is close to the maximum T c of 108 K expected of the optimum of Bi-2223 system. The width of the superconducting phase transition is narrower, suggesting the investigated Bi-2223 sample to be uniform.
4 Conclusions
Lattice parameters determined from the XRD peaks were a=5.4054 Å, b=5.4111 Å and c=37.0642 Å for Bi-2223. The surface morphology i.e. pores, voids, size of grains, and their orientation etc. are varying, however, the resulting average cationic compositions of pellets were found in 2:2:2:3 ratios in almost each region. The two-dimensional AFM image appears as a nanosphere like structure on the surface and the most pronounced difference in the two layers of oxide is around 37.673 nm. The transition temperature (T c ) was found ∼108 K for Bi-2223. An important microstructural/structural characteristic found in all of the double and triple CuO2 layer Bi-bearing HTSC compounds is the presence of weak super lattice reflections in the selected area electron diffraction patterns. The ZFC and FC curves of the Bi-2223 sample are found after final sintering at 848∘C.
References
Ozturk, O., Yegen, D., Yilmazlar, M., Varilci, A., Terzioglu, C.: Physica C 451, 113–117 (2007)
Terzioglu, C., Audin, H., Ozturk, O., Bekiroglu, E., Beleni, I.: Physica B 403, 3354–3359 (2008)
Gul, I.H., Amin, F., Abbasi, A.Z., Rehman, M.A., Maqsood, A.: Physica C 449, 139–147 (2006)
Sozeri, H., Ghazanfari, N., Ozkan, H., Kilic, A.: Supercond. Sci. Technol. 20, 522–528 (2007)
Gul, I.H., Rehman, M.A., Ali, M., Maqsood, A.: Physica C 432, 71–80 (2005)
Bilgili, O., Selamet, Y., Kocabas, K.: J. Supercond. Nov. Magn. 21, 439–449 (2008)
Gul, I.H., Rehman, M.A., Maqsood, A.: Physica C 450, 83–87 (2006)
Lee, W.H., Wu, P.T., Chen, Y.C., Liu, R.S., Liang, J.M., Chen, L.J., Klavius, P., Shelton, R.N.: Physica C 152, 345–352 (1988)
Jeremic, A., Grasso, G., Flu Kinger, R.: Physica C 225, 53–58 (1995)
Chen, F.H., Koo, H.S., Tseng, T.Y., Liu, R.S., Wu, P.T.: Mater. Lett. 8, 228–232 (1989)
Suvarna, A., Sunandanatt, C.S.: Solid State Commun. 95, 11–16 (1995)
Paz Pujalt, G.R.: Physica C 166, 177–184 (1990)
Pignon, B., Veron, E., Noudem, J., Ruyter, S., Ammor, L., Monot-Laffez, I.: Physica C 434, 45–52 (2006)
Acknowledgements
We are grateful to Prof. O.N. Srivastava and Prof. R.S. Tiwari of Department of Physics, B.H.U., Varanasi for helpful and stimulated discussions. One of the authors (Dr. B. Das) acknowledges financial support from University Grant Commission, Government of India as a major research project (F.No. 36-263/2008 (SR)).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Verma, I., Kumar, R., Ganesan, V. et al. Synthesis and Magnetic Properties of (Bi, Pb)2Sr2Ca2Cu3O10+δ Superconductor. J Supercond Nov Magn 25, 785–789 (2012). https://doi.org/10.1007/s10948-011-1339-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-011-1339-6