Abstract
Stable oxygen isotope measurements on fossil chironomid head capsules from lake sediments show that these chitinous remains can be used to reconstruct past lake water δ18O and, indirectly, past climate change. We examined the impact of chemical pretreatment procedures on the chemical and stable oxygen isotope composition, and morphology of chironomid cuticles. Use of alkali, acids, and sodium chlorite alters the chemical composition and the morphological structure of chironomid cuticles by selective removal of chitin or proteins. Gas chromatograms of pyrolyzates show that NaClO2 causes deproteination, whereas the combined use of HCl and HF results in partial chitin removal. Head capsules pretreated with KOH contained both chitin- and protein-derived moieties, although the concentration of protein was reduced, especially after KOH treatment at high concentration (28%) and temperature (100°C). Scanning electron microscopy confirmed that a proteinaceous matrix is still present in modern and fossil head capsules after KOH treatment. This matrix, however, is largely absent in head capsules pretreated with NaClO2. A change in the proportion of chitin and proteins in our samples was associated with differences in chironomid δ18O values. Our results suggest that deproteination results in a relative increase of chironomid δ18O, whereas removal of chitin leads to decreased δ18O values. We therefore discourage the use of acids or prolonged (≥1 h) exposure to hot alkali (70°C) prior to chironomid δ18O analysis. Chitin purification by sodium chlorite causes significant weight loss, which may preclude down-core chironomid δ18O measurements. Caution and standardization are required when pretreating samples for chironomid δ18O analysis to ensure reliable, comparable, and reproducible results.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Uncertainties about impacts of future environmental change, including global warming, have led to growing public concern. Understanding past climate variations is important for assessing the potential impacts of future climatic changes. Lakes preserve a record of environmental change in their sediments, which can be used to infer climate changes in the pre-instrumental period (Smol 2008). A number of biotic and abiotic variables in lake sediments provide valuable information on past environmental changes (Battarbee 2000; Von Gunten et al. 2008; Bjune et al. 2009; Moore et al. 2001; Loso 2009). Although some of these proxy variables have been applied successfully to reconstruct past climate shifts, considerable effort is required to develop accurate temperature indicators. Temperature proxies are most reliable if applied within a multi-proxy framework (Lotter 2003; Birks and Birks 2006). Application of multiple temperature indicators, however, is limited to certain regions, lakes, or sediment types, and although a few methods can be used universally (Blaga et al. 2009), they often apply only to the warm season.
Stable oxygen isotope analysis of lacustrine carbonates, such as shells of molluscs or ostracods, is a common approach for reconstructing past lake water isotopic composition and for obtaining information on past temperature changes (Von Grafenstein et al. 1999; Schwalb 2003; Leng and Marshall 2004). Furthermore, δ18O in bulk carbonate is an excellent tool for correlating regional lake sediment records during phases of distinct climatic changes (Lotter et al. 1992). Many lake sediments, especially those from silicate bedrock regions, do not contain carbonates. In such cases, isotopic measurements on other autochthonous substances preserved in lake sediments provide an alternative approach for reconstructing past lake water δ18O. For instance, several methods have been developed to extract, e.g. algal cellulose from sediments for δ18O analysis (Wolfe et al. 2001; Wissel et al. 2008). Chitin, a component of exoskeletons of many aquatic invertebrates, is another common biomacromolecule in lake sediments. Chitin (poly-N-acetyl-d-glucosamine; Fig. 1) is a biopolymer that preserves well under suitable conditions (Muzzarelli 1977), such as environments characterized by high sedimentation rates (Van Waveren 1994), high productivity, and reducing conditions in bottom waters (Stankiewicz et al. 1997a). Chitin in insect cuticles is thought to be cross-linked with proteins through quinonoid derivatives of catecholamines (Schaefer et al. 1987). Besides chitin and proteins, lipids and pigments constitute a minor part of insect cuticles (Richards 1978).
Chemical structure of poly-N-acetyl-d-Glucosamine (chitin) linked to proteins in insect cuticles through catecholamines and histidine moieties (modified after Schaefer et al. 1987)
Chironomids (non-biting midges; Diptera: Chironomidae) are insects whose larvae are abundant in lakes. Their exoskeletons contain chitin. Chironomid larvae live in benthic freshwater environments and undergo several molts (ecdysis) during which the head capsule is shed (Hopkins and Kramer 1992). Both head capsules released during ecdysis and those from deceased specimens are usually well preserved in lake sediments. Past environments have been inferred from changes in the taxonomic composition of fossil chironomid assemblages, using information on the ecology and distribution of different taxa (Brooks 2006; Walker and Cwynar 2006; Heiri et al. 2007; Engels et al. 2008). Wooller et al. (2004, 2008) and Wang et al. (2008) demonstrated the use of chitinous chironomid head capsules to generate δ18O records, which have great potential for palaeotemperature reconstruction. The effects of chemical pretreatment procedures on chironomid δ18O, however, still must be fully evaluated. Detailed information on changes in the chemical composition and δ18O of chironomid head capsules associated with different chemical pretreatments will allow further development of this method and will reduce uncertainties associated with δ18O analysis.
In the 1980s and 1990s the δ18O composition of purified arthropod chitin was analyzed in several studies (Schimmelmann and DeNiro 1985, 1986a, b; Schimmelmann et al. 1986) and the chemical composition of chitinous insect cuticles was characterized (Van der Kaaden et al. 1984; Schaefer et al. 1987; Kramer et al. 1995; Stankiewicz et al. 1996, 1997a, b, c, 1998; Briggs et al. 1998). Schimmelmann and DeNiro (1986a) recommend purifying chitin in modern and fossil cuticles to d-glucosamine hydrochloride (GlcN.HCl; Schimmelmann and DeNiro 1986b). However, d-glucosamine preparation and extraction is labor-intensive and may cause contamination by introduction of non-authigenic oxygen. Moreover, purification leads to weight loss and may eventually yield a sample too small to be measured (Hodgins et al. 2001). Wooller et al. (2004) completed a study of how various chemicals influence chironomid δ18O, but did not detect any effect, and a protocol was proposed for preparing chironomid remains for δ18O measurement, using alkali and acids (Wang et al. 2008). We studied the effects of several commonly used chemicals (e.g. KOH, HF, and HCl) on the chemical composition and δ18O of chironomid head capsules. Because chitin is similar to cellulose, differing only in the presence of N-acetyl groups in chitin, a method developed to process and purify cellulose samples prior to δ18O analysis (Leavitt and Danzer 1993) was also tested. We used scanning electron microscopy (SEM) to detect morphological changes in head capsule cuticles. In this study, we addressed the following questions: (a) what effects do the various pretreatments have on the relative abundances of chitin and proteins in chironomid head capsules, and on the morphology of their cuticles? and (b) what are the implications of changes in chemical composition for chironomid δ18O analysis? Sorting chironomid head capsules from lake sediments under a dissecting microscope is very labor intensive. We thus determined the minimum chironomid sample weight that can be used to produce a reliable isotopic measurement and we discuss the suitability of the different chemical pretreatment methods in the context of the minimum weight necessary for δ18O analysis.
Materials and methods
Chironomid (Chironomus riparius) larvae, commonly used as fish food, were obtained from a commercial source (Discusfarm Marsilea, Lelystad, The Netherlands). To guarantee preservation, frozen larvae were obtained. We assume that the freezing process did not affect the chemical and stable oxygen isotopic composition, or morphology of the head capsules. A purified chitin standard (Sigma–Aldrich, C9752, St. Louis, MO, USA), derived from marine crab shells, was compared with the chemical composition of chironomid head capsules. Pretreatment chemicals were added to the head capsules in a 100-ml glass beaker containing a stirring magnet, unless indicated otherwise. Fossil chironomid head capsules from the sediments of Rotsee, Switzerland, which date to ~16,000 cal years BP (Lotter and Zbinden 1989), were used for comparison with fresh head capsules.
Pretreatments
Palaeoecological analyses often involve chemical pretreatment of sediments. KOH, HCl, and HF are commonly used. They alter the sample pH and may induce changes in chemical composition of organic materials. We tested the effects of these chemicals on the morphology, as well as the chemical and stable oxygen isotopic composition of chironomid head capsules. We also evaluated a method that is used to purify cellulose prior to δ18O analysis.
Demineralized water pretreatment
A batch of chironomid larvae was treated with demineralized water for 24 h at room temperature (~20°C) to represent chemically untreated chironomid head capsules. Larval head capsules were subsequently removed from the bodies under a stereo microscope using forceps. Digestive tracts and muscle tissue were carefully detached from the head capsules to avoid contamination by organic material in the larval gut. Head capsules containing sandy detritus were eliminated from further analysis.
Alkaline pretreatments
Potassium hydroxide (KOH) is often used to deflocculate sediment organic material prior to sieving and is used in the standard preparation of samples for microscopic identification of fossil chironomids (Walker 2001; Brooks et al. 2007). This alkaline treatment causes deproteination of insect cuticles (Schimmelmann et al. 1986; Einbu and Vårum 2008). Because this process accelerates with increasing temperature, the effect of different temperatures and exposure times on head capsule composition was examined. Larvae were soaked in 10% KOH for 2 h at room temperature (~20°C) or for 1 h at 70°C. An additional batch of larvae was soaked in 28% KOH for 24 h at 100°C. After exposure to KOH, larvae were rinsed 10 times with demineralized water and head capsules were removed manually under a dissecting microscope.
Acid pretreatment
Hydrochloric acid (HCl) and hydrofluoric acid (HF) are commonly used to remove carbonates and other minerals (e.g. silicates), respectively. Acids may induce exchange of OH groups (Roberts and Urey 1939). This exchange may lead to δ18O values that do not reflect the original signal in the head capsules. We analyzed chironomid larvae that were exposed to 30% HCl, and subsequently to 40% HF, for 1 and 2 h at room temperature, respectively, in a plastic pot on a shaker apparatus. After acid treatment, larvae were washed thoroughly with demineralized water. Head capsules were subsequently removed from larval bodies as described above.
Sodium chlorite pretreatment
Head capsules were pretreated using a common method to purify cellulose for δ18O analysis. This method, modified from the “Jayme-Wise” technique (Green 1963), is described in detail by Leavitt and Danzer (1993) and will be referred to as the LD method. The method involves transfer of head capsules to glass fiber filters, followed by accelerated solvent extraction (ASE) using a mixture of dichloromethane and methanol (9:1) to remove waxes, oils, and resins. This first step is followed by boiling in deionized water for 6 h to remove inorganic salts and low-molecular-weight polysaccharides. Next, several additions of sodium chlorite and glacial acetic acid are used to purify the chitin. Finally, samples are washed multiple times with demineralized water and oven dried over night at 60°C.
Curie-point pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS)
Flash pyrolysis allows macromolecular organic substances to be characterized by their low molecular weight pyrolysis products, after rapid heating in an inert environment. The chemical composition of head capsules and chitin can be assessed by examining mass spectrometric fragment ions that characterize components reflected as peaks in the chromatograms. Samples were pressed onto a flattened ferromagnetic wire and pyrolyzed at 610°C for 10 s in a continuous He flow using a curie point pyrolyzer (FOM) coupled directly to a Thermo Finnigan Trace GC Ultra gas chromatograph, interfaced to a Thermo Finnigan Trace DSQ mass spectrometer. Compounds were separated using a silica capillary column with an inner diameter of 0.32 mm coated with a 0.4-μm film (Varian, CP-Sil-5CB). The oven was programmed from 40°C (constant for 5 min) to 230°C at a rate of 3°C/min and further increased to 300°C at a rate of 20°C/min, maintaining this endpoint temperature for 10 min. The MS was operated in full-scan mode (m/z 50–800, 2.5 scans/s, 70 eV electron energy, 250°C source temperature). Compounds were identified by their retention times and mass spectra using literature data (Van der Kaaden et al. 1984; Boon and De Leeuw 1987; Chiavari and Galletti 1992; Stankiewicz et al. 1996, 1997a, b, 1998; Bierstedt et al. 1998; Flannery et al. 2001).
Stable oxygen isotope analysis (TC-EA/IRMS)
A high-temperature conversion elemental analyzer (TC-EA; Thermo Finnigan) coupled to an isotope ratio mass spectrometer (IRMS; Thermo Finnigan Deltaplus) was used to determine ratios of stable oxygen isotopes of organic substances in relatively small amounts (50–100 μg range; Kornexl et al. 1999). Both head capsules and a chitin standard (Sigma–Aldrich) were used to assess the minimum weight needed for δ18O analysis. All samples were analyzed using 4 mm × 3.2 mm silver cups (Elemental Microanalysis Ltd, batch number 128843). Two blanks were measured at the start of every run. Standardization was achieved using a cellulose standard (IAEA-C3), benzoic acid standard (HEKAtech, batch number 33822501), and two international potassium nitrate standards (IAEA-NO-3 en USGS-32). δ18O data are reported in per mille (‰) relative to the V-SMOW (Vienna Standard Mean Ocean Water) standard (Coplen 1996). Two outliers were detected using Grubbs’ test and were removed from the data set. Contamination probably explains these outliers. Only measurements that passed strict data quality control criteria for minimum voltage (>950 mV) and stable backgrounds were used for data interpretation.
Scanning electron microscopy (SEM)
Morphology of the cuticles of chironomid head capsules subjected to the various pretreatments was studied by scanning electron microscopy (SEM). Head capsules were cut into smaller fragments and rinsed in demineralized water before mounting. Fragments were mounted onto negative film attached to a stub with Araldite in such a way that the inner structures of the cuticles (i.e. perpendicular to the surface) were displayed. Fragments were coated with 12 nm platinum using a sputter coater (Cressington 208 h), and examined under a SEM (Philips XL30S FEG) at 65,000× magnification.
Results
Pyrolysis-GC/MS
Pretreatment effects
The impact of the various pretreatments on the chemical composition of chironomid head capsules was deduced from the chromatograms of the pyrolyzates in Fig. 2. Pyrolysis products are reflected by peaks in these chromatograms and are identified based on their characteristic mass spectra (Table 1). All compounds are derived from either chitin or proteins. 1,6-Anhydro-2-acetamido-2-deoxyglucose (28) is the most abundant pyrolysis product and shows up as a high and broad peak in most chromatograms.
Total ion current traces obtained by Curie-point pyrolysis-gas chromatography mass spectrometric analysis of chironomid head capsules pretreated 24 h in demineralized water at 20°C (a), 2 h in KOH 10% at 20°C (b), 1 h in KOH 10% at 70°C (c), 1 h in HCl 30% and 2 h in HF 40% at 20°C (d) and in sodium chlorite (Leavitt and Danzer 1993 method; e), as well as of crab chitin standard (Sigma–Aldrich; f). Numbers indicate peaks characteristic of chitin and letters mark peaks indicative of proteins
The chromatograms indicate that the proportion of chitin and protein in chironomid head capsules varies with pretreatment. Many chitin-derived compounds are present in head capsules that were treated with NaClO2, as well as in the crab chitin standard. However, some of these components, such as acetylpyridone (17) and N-hydroxyphenylacetamide (21) are not observed in the chromatograms of the head capsules treated with demineralized water or KOH. Even fewer chitin-derived compounds are seen in the acid-treated samples. On the other hand, only a fraction of the protein-associated peaks that are recognized in the chromatograms of head capsules pretreated with demineralized water, alkali, or acids are present in the chromatograms of head capsules that underwent the LD pretreatment and of crab chitin.
Although Py-GC/MS cannot quantify absolute concentrations, differences in the areas under various peaks allows estimation of relative abundances of compounds in head capsules. A chitin:protein ratio was calculated to infer quantitative changes in relative abundances of chitin to proteins. This ratio was calculated by dividing the sum of areas of compounds 28 and 28′, i.e. dominant chitin-derived compounds, by the sum of areas of compounds i and j, i.e. dominant protein-derived components. A ratio of 1 indicates equal abundances of chitin and protein-derived pyrolysis products. The lowest ratio was found in the head capsules pretreated with acids, followed by head capsules treated with demineralized water, 2 h KOH 10% at 20°C, and 1 h KOH 10% at 70°C (Table 2). Head capsules pretreated with the LD method consist almost entirely of chitin.
The LD-pretreated head capsules resemble purified crab chitin in that both contain few protein-derived pyrolysis products. Differences among the chromatograms from head capsules pretreated with KOH, acids, or demineralized water, are relatively small, indicating a similar mixture of chitin and proteins. Head capsules pretreated with HCl and HF, however, contain relatively low abundances of pyrolysis products indicative of chitin, and the highest amount of pyrolysis products derived from proteins. The presence of many acetylated pyrolysis products suggests that chitin in crab chitin and chironomid head capsules is not entirely deacetylated to chitosan.
Fossil head capsules
Fossil head capsules from late glacial sediment of Rotsee, Switzerland, were also analyzed using Py-GC/MS, and their composition was compared with pretreated head capsules. These ~16,000-year-old fossil head capsules contain both abundant chitin- and protein-derived compounds (Fig. 3b). Pyrolysis products are most similar to those in head capsules pretreated with 10% KOH for 1 h at 70°C, indicating a similar chemical composition.
Total ion current traces of the Curie-point pyrolysis-gas chromatography mass spectrometric analysis of modern chironomid head capsules pretreated with 10% KOH for 1 h at 70°C (a) and fossil chironomid head capsules isolated from Rotsee sediments of late glacial age (~16,000 cal years BP) (b). Numbers and letters refer to peaks derived from chitin and proteins, respectively (note the different scales of y-axes)
δ18O analysis
Minimum weight assessment
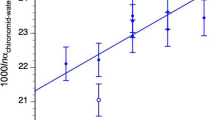
For chironomid head capsules, as well as for both the chitin and cellulose standards, the minimum sample size necessary for δ18O analysis was determined by analyzing samples of weights ranging from 4 to 250 μg (Fig. 4). Samples display stable δ18O values when weights are >50 μg. This limit corresponds to a voltage of 950 mV and was adopted as the minimum weight for chironomid δ18O analysis using our equipment.
Pretreatment impact
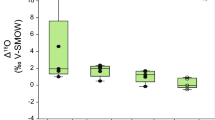
We evaluated effects of pH-neutral, alkaline, and acidic pretreatments, as well as the LD pretreatment on δ18O values of chironomid head capsules (Fig. 5). Head capsules exposed to 10% KOH for 1 h at 70°C were taken as a matrix-matched reference and used for inter-calibration of δ18O values. This pretreatment was chosen as a reference because it facilitates removal of head capsules from larval bodies, eliminates soft body tissue, and therefore mimics the fossilization process. The inter-calibration is done to compensate for possible drift in δ18O values for every run, and changes with respect to this reference are shown in Fig. 5. Dashed and dotted lines represent the 1- and 2-σ range of reference δ18O values, respectively. Different pretreatments produced statistically significant differences in δ18O values in all cases (two-tailed t-test, α = 0.05), except for the groups pretreated with acids and KOH 10% for 1 h at 20°C (P = 0.11; Table 3).
Effects of various pretreatments involving alkali (diamonds, squares, triangles), acids (crosses), demineralized water (x-symbols) and sodium chlorite (circles) on the stable oxygen isotopic composition of chironomid head capsules (1 h 10% KOH 70°C measurements are used as reference). Y axis shows differences in δ18O values with respect to reference. Dashed and dotted lines delimit the ±1 and 2 standard deviation range of the reference, respectively
The higher the temperature applied during KOH pretreatment, the higher the δ18O offset, leading to increased chironomid δ18O (Fig. 5). The use of acids, however, removes isotopically heavier components and/or causes isotope exchange between oxygen in cuticles and the water in which the acid is dissolved, which results in relatively depleted δ18O values for head capsules. The LD method results in relatively 18O-enriched head capsules. This enrichment is not an artifact of adherence of glass fiber filters to the head capsules during the LD pretreatment, as filters were measured separately for δ18O and found to contain too little oxygen to interfere with chironomid δ18O measurements.
Head capsule morphology
We detected differences in head capsule morphology for the different treatments by examining fractured edges of chironomid head capsules under the SEM (Fig. 6). Insect cuticles consist mainly of multiple overlapping layers of chitin fibers, or rods, embedded in a protein matrix (Nation 2002). This is seen clearly in cuticles of head capsules pretreated with demineralized water (Fig. 6a), whereas this matrix is slightly reduced in fossil head capsules (Fig. 6f). The matrix is, to a lesser extent, apparent in head capsules pretreated with KOH (Fig. 6b, c) and acids (Fig. 6d), where the ‘plywood’ structure that is typical for insect exoskeletons (Nation 2002) can clearly be distinguished. However, it seems to be absent in cuticles pretreated with the LD method (Fig. 6e). In the latter case, long fibers protrude from the fractured cuticle edges. SEM analyses thus indicate that cuticles are morphologically altered by pretreatment.
SEM images of fractured edges of chironomid head capsule cuticles pretreated with demineralized water for 24 h at 20°C (a), KOH for 2 h at 20°C (b), KOH for 1 h at 70°C (c), HCl 1 h and HF 2 h (d), sodium chlorite (Leavitt and Danzer (1993) method; e), as well as of a fossil chironomid head capsule from the sediment of Rotsee (f)
Discussion
We analyzed the chemical composition of chironomid head capsules and showed that chitin moieties are present in head capsule cuticles, regardless of pretreatment and age in this case. Often the most dominant pyrolysis product of chitin was 1,6-anhydro-2-acetamido-2-deoxyglucose (28), which was present in every pyrolyzate (Figs. 2, 3). It is the primary pyrolysis product of the intact monomer, or building block of chitin, and is produced by depolymerization of chitin, followed by dehydration (Van der Kaaden et al. 1984). Both pyrolysis results and SEM images show that pretreatments have an effect on the relative amount of chitin and proteins present in chironomid head capsules, which in turn affects cuticle δ18O values. Relative abundances of chitin and proteins in head capsules pretreated with either demineralized water or KOH are similar (Figs. 2a–c), although the chitin:protein ratio is higher in the hot alkali pretreatment (Table 2). The presence of pyrolysis products of proteinaceous origin indicates that amino acids such as tyrosine (characterized by pyrolysis products such as phenols), tryptophan (indoles), phenylalanine (toluene), and proline (pyrroles) are, to some extent, resistant to these pretreatments. This implies that deproteination caused by base hydrolysis did not occur extensively during either the hot or cold alkali pretreatment. This is in agreement with Brine and Austin (1981), who exposed horseshoe crab shells to 1 M NaOH for 6 h at 50°C and found proteins after treatment. The fact that little deproteination occurs is also evident from the SEM images (Figs. 6a–c), in which sheets of chitin fibers are seen embedded in a proteinaceous matrix in the typical ‘plywood’ structure of the insect exocuticle (Giraud-Guille and Bouligand 1986; Nation 2002). Enriched δ18O values after KOH pretreatment at higher temperatures suggest that temperature enhances the reaction between KOH and head capsules, whereas the length of treatment was less important in our experiments. It also implies that material removed using KOH has a lighter δ18O value than the remaining material.
Acid pretreatment leads to a substantial decrease in the amount of head capsule chitin, as seen in the low chitin:protein ratio (0.19; Table 2). Only a few chitin-derived compounds, such as anhydrosugars, remain in the pyrolyzates at low relative abundances. Acids are known to catalyze the loss of N-acetyl groups (de-N-acetylation; Einbu and Vårum 2007). De-N-acetylation is therefore thought to be the mechanism behind this chitin decrease. If de-N-acetylation occurs extensively, chitin is partly converted to chitosan, which is soluble in acidic aqueous solutions (Pariser and Lombordi 1980). If most chitosan is dissolved, only a small amount of chitin remains relative to proteins. This is reflected in the reduced relative abundances of chitin-derived compounds in the chromatogram of acid-treated head capsules (Fig. 2d) and the relatively low abundance of chitin fibers observed in the SEM image (Fig. 6d). Furthermore, acids induce exchange of oxygen between chitin and water. Because the δ18O of head capsules (~16‰) is heavier than that of the water used (~−7‰), head capsules soaked in acids end up isotopically lighter due to oxygen exchange (Fig. 5).
Head capsules pretreated with the LD method contain many chitin-derived compounds and only a few compounds associated with proteins (Fig. 2e). The chitin:protein ratio, which is calculated using protein pyrolysis products i and j, cannot be determined due to the absence of these compounds. Substantial deproteination is attributed to the use of NaClO2. The resultant morphological change is visible in the SEM image (Fig. 6e), in which chitin rods are seen protruding from a fractured edge of an acid-treated head capsule cuticle that lacks the proteinaceous matrix.
The pyrolyzate of purified crab chitin standard contains only one minor pyrolysis product derived from protein moieties (Fig. 5f), and therefore consists predominantly of chitin, which confirms the findings by Stankiewicz et al. (1996). Our results show that chemical pretreatment can cause selective removal of chitin and/or protein moieties.
The chemical stability of chitin and persistence of proteins is demonstrated by their abundance in fossil chironomids of late glacial age (Fig. 3b). Traces of chitin have even been found in 25-Ma-old insect remains from lacustrine deposits of the Enspel Formation, Germany (Stankiewicz et al. 1997a; Gupta et al. 2007). Although chitin can be degraded both aerobically (Boyer 1994; Reguera and Leschine 2001) and anaerobically (Sturz and Robinson 1986), the depositional environment is thought to play a prominent role in the molecular preservation of both chitin and proteins (Stankiewicz et al. 1997a, 1998; Briggs et al. 1998; Gupta et al. 2007). Lakes may provide a suitable environment to preserve these biopolymers on long timescales. Although proteins are more susceptible to degradation than chitin (Baas et al. 1995; Briggs et al. 1998), their pyrolysis products are still present in the late glacial chironomids as well as in the Miocene beetles studied by Stankiewicz et al. (1997a). Fossil chironomids analyzed here mostly resemble the head capsules pretreated with demineralized water or KOH, with respect to their chemical composition and morphology (Figs. 3, 6). Distributions of pyrolysis products and the chitin:protein ratio in fossil head capsules correspond most closely to those of head capsules pretreated with KOH (Tables 1, 2; Fig. 3).
Relative amounts of chitin in extant insect cuticles range between 20 and 50% (Hackman 1974; Muzzarelli 1977; Andersen 1979; Kramer et al. 1995; Bierstedt et al. 1998). The proportion of chitin in insects from Pleistocene sediments is usually between 10 and 30% (Miller et al. 1993), but can approach 40% even in Pliocene deposits (Bierstedt et al. 1998). Although pyrolysis does not allow absolute quantification of the amount of chitin, the estimated chitin:protein ratio of ~1 in fossil chironomid head capsules (Table 2) indicates that the chitin proportion in the sample is in agreement with the literature.
Pretreatment effects on chironomid δ18O
Different pretreatments have different effects on the stable oxygen isotopic composition of chironomid head capsules (Fig. 5). Progressive deproteination, as seen in chromatograms and SEM images of the head capsules pretreated with the LD method, as well as in head capsules exposed to prolonged hot alkali pretreatment (28% KOH for 24 h at 100°C), causes enrichment of δ18O values. On the other hand, head capsules that experience removal of chitin relative to proteins, such as those processed by acids, display depleted δ18O values. Two main processes may contribute to these shifts in isotope values. First, oxygen isotope exchange induced by the use of acids alters the δ18O of head capsules toward the δ18O value of the water in which the acid is dissolved, thus leading to a depletion in the δ18O value of the head capsule in our study. Second, fractionation processes that occur when the chironomid exoskeleton is formed affect protein and chitin components of head capsules differently. Proteins and chitin are formed via different biochemical pathways (Nation 2002), which may explain differences in oxygen isotope fractionation. Our experiments suggest that pretreatments that cause deproteination may produce head capsules with up to 5–6‰ heavier δ18O values (Fig. 5). Acid pretreatment, on the other hand, results in selective preservation of proteins as opposed to chitin, but also induces oxygen isotope exchange, the combination of which leads to significant oxygen isotopic depletion (2‰) in head capsules. Currently, we cannot assess the relative contribution of oxygen isotope exchange versus compound-specific fractionation, to this oxygen isotope shift. Nevertheless, δ18O enrichment of head capsules that have undergone progressive deproteination in a pH-neutral or alkaline environment, where no oxygen isotope exchange has taken place, suggests that chitin is isotopically heavy with respect to proteins, and that selective chitin removal in the acid treatment is an important factor causing changes in δ18O.
Wooller et al. (2004, 2008) demonstrated that chironomid δ18O is in equilibrium with lake water δ18O and therefore has the potential to be used as a palaeotemperature proxy. Wang et al. (2008) recently proposed a protocol for chironomid pretreatment prior to δ18O analysis, including treatments with 10% HCl for 24 h and 5% KOH for 15–20 min at 60–70°C. The advantage of their two-step approach is that chironomid samples, once prepared, can be stored before final transfer to silver cups for TC-EA/IRMS analysis. Wang et al. (2008) used weak to moderate alkali (KOH) and acid (HCl) treatments. We demonstrated that prolonged exposure to hot alkali, and even more so, use of acids, alters the chemical composition and stable oxygen isotopic composition of head capsules. When applied in paleolimnological studies, down-core shifts in chironomid δ18O are still observed, even if the values are affected by a systematic bias. Reconstructions of the past oxygen isotopic composition of lake water, however, may be obscured by the biases caused by such chemicals. This problem may be minimized by performing alkali and acid pretreatments under closely controlled conditions, that is, by using consistent sample exposure times and acid/alkali concentrations, and using water with the same δ18O composition. Nevertheless, because acid treatment has been shown to promote exchange of oxygen atoms, at least in organic material derived from algal biomass (Wedeking and Hayes 1983), we strongly discourage the use of acids.
Proteins are thought to have a different stable oxygen isotopic composition than chitin, and their δ18O value is also affected by their amino acid composition. A possible preparation procedure might be to isolate the chitin component of the head capsules before analysis. The LD method seems a suitable method for chitin purification. This processing step, however, leads to major weight loss in samples, >45% in our experiments. Hodgins et al. (2001) applied labor-intensive preparation approaches to purify chitin for dating purposes and found similar yields after deproteination of insect chitin. Such a weight loss may preclude using chironomid δ18O down-core, because chironomid concentrations are often low. Even in lakes with very high head capsule concentrations, production of high-resolution records may be difficult given the long time required to sort sufficient chironomid remains from the sediments. The use of chemicals similar to those investigated in this study apparently does not alter the δ13C of chironomid head capsules (Van Hardenbroek et al. in press).
Conclusions
We assessed the effect of chemical pretreatments that are commonly used in paleolimnology, on the chemical composition, δ18O, and morphology of chironomid head capsules. The chemical composition and morphology of late glacial chironomid fossils from Rotsee are very similar to head capsules of extant chironomid larvae that were exposed briefly to alkali, or exposed for longer duration to demineralized water. This suggests that extensive protein and chitin degradation did not occur during the fossilization process. Presence of the chitin monomer in pyrolyzates of all studied samples confirms the chemical stability of chitin, regardless of sample age or chemical pretreatment.
Whereas cold alkali pretreatments have little effect on chironomid remains, prolonged (≥1 h) exposure to hot alkali, acids, or NaClO2 (LD method) alters their chemical and stable oxygen isotope composition by oxygen isotope exchange, and/or removal of proteins or chitin, two main components of chironomid head capsules. Selective removal of these components introduces a bias in the δ18O signal in head capsules, which is thought to reflect past lake water δ18O, and, hence, past temperatures. We, therefore, strongly discourage the use of acids or prolonged exposure to hot alkali in preparing chironomids for δ18O analysis. Purification of chitin from head capsules in a pH-neutral or alkaline environment does not alter the δ18O signal in the purified chitin, and probably yields the most reproducible chironomid δ18O records. The purification process, however, causes significant sample weight loss, thereby precluding the use of this approach for developing high-resolution, down-core records. Because different pretreatments alter the δ18O of chironomid remains in different ways, it will be necessary to calibrate separately the relationship between δ18O of chironomids treated with acids, hot alkali, or less aggressive pretreatment methods, and lake water δ18O values. Given the differential effects of various preparation techniques on the chemical and oxygen isotope signature of chironomid head capsules, care must be taken when comparing chironomid δ18O records that used different pretreatment methods.
References
Andersen SO (1979) Biochemistry of insect cuticle. Annu Rev Entomol 24:29–61
Baas M, Briggs DEG, Van Heemst JDH, Kear AJ, De Leeuw JW (1995) Selective preservation of chitin during the decay of shrimp. Geochim Cosmochim Acta 59:945–951
Battarbee RW (2000) Palaeolimnological approaches to climate change, with special regard to the biological record. Quat Sci Rev 19:107–124
Bierstedt A, Stankiewicz BA, Briggs DEG, Evershed RP (1998) Quantitative and qualitative analysis of chitin in fossil arthropods using a combination of colorimetric assay and pyrolysis-gas chromatography-mass spectrometry. Analyst 23:139–145
Birks HH, Birks HJB (2006) Multi-proxy studies in palaeolimnology. Veg Hist Archaeobot 15:235–251
Bjune AE, Seppä H, Birks HJB (2009) Quantitative summer-temperature reconstructions for the last 2000 years based on pollen-stratigraphical data from northern Fennoscandia. J Paleolimnol 41:43–56
Blaga CI, Reichart G-J, Heiri O, Sinninge Damsté JS (2009) Tetraether membrane lipid distributions in water-column particulate matter and sediments: a study of 47 European lakes along a north–south transect. J Paleolimnol 41:523–540
Boon JJ, De Leeuw JW (1987) Amino acid sequence information in proteins and complex proteinaceous material revealed by pyrolysis-capillary gas chromatography-low and high resolution mass spectrometry. J Anal Appl Pyrol 11:313–327
Boyer JN (1994) Aerobic and anaerobic degradation and mineralization of 14C-chitin by water column and sediment inocula of the York River estuary, Virginia. Appl Environ Microb 60:174–179
Briggs DEG, Stankiewicz DA, Meischner D, Bierstedt A, Evershed RP (1998) Taphonomy of arthropod cuticles from Pliocene lake sediments, Willershausen, Germany. Palaios 13:386–394
Brine CJ, Austin PR (1981) Chitin variability with species and method of preparation. Comp Biochem Physiol B 69:283–286
Brooks SJ (2006) Fossil midges (Diptera: Chironomidae) as palaeoclimatic indicators for the Eurasian region. Quat Sci Rev 25:1894–1910
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of Palaearctic Chironomidae larvae in palaeoecology. Quat Res Assoc Tech Guide 10:1–276
Chiavari G, Galletti GC (1992) Pyrolysis-gas chromatography/mass spectrometry of amino acids. J Anal Appl Pyrol 24:123–137
Coplen TB (1996) New guidelines for reporting stable hydrogen, carbon, and oxygen isotope-ratio data. Geochim Cosmochim Acta 60:3359–3360
Einbu A, Vårum KM (2007) Depolymerization and de-N-acetylation of chitin oligomers in hydrochloric acid. Biomacromolecules 8:309–314
Einbu A, Vårum KM (2008) Characterization of chitin and its hydrolysis to GlcNAc and GlcN. Biomacromolecules 9:1870–1875
Engels S, Bohncke SJP, Bos JAA, Brooks SJ, Helmens KF (2008) Chironomid-based palaeotemperature estimates for northeast Finland during oxygen isotope stage 3. J Paleolimnol 40:49–61
Flannery MB, Stott AW, Briggs DEG, Evershed RP (2001) Chitin in the fossil record: identification and quantification of d-glucosamine. Org Geochem 32:745–754
Giraud-Guille MM, Bouligand Y (1986) Chitin-protein molecular organization in Arthropods. In: Muzzarelli R, Ch Jeuniaux, Gooday GW (eds) Chitin in nature and technology. Plenum Press, New York, pp 29–35
Green JW (1963) Wood cellulose. In: Whistler RL (ed) Methods in carbohydrate chemistry, vol III. Academic Press, New York, pp 9–21
Gupta NS, Briggs DEG, Collinson ME, Evershed RP, Michels R, Pancost RD (2007) Molecular preservation of plant and insect cuticles from the oligocene enspel formation, Germany: evidence against derivation of aliphatic polymer from sediment. Org Geochem 38:404–418
Hackman RH (1974) In: Rockstein M (ed) The physiology of insecta. Academic Press, New York, pp 215–270
Heiri O, Cremer H, Engels S, Hoek WZ, Peeters W, Lotter AF (2007) Lateglacial summer temperatures in the Northwest European lowlands: a chironomid record from Hijkermeer, The Netherlands. Quat Sci Rev 26:2420–2437
Hodgins GWL, Thorpe JL, Coope GR, Hedges REM (2001) Protocol development for purification and characterization of sub-fossil insect chitin for stable isotopic analysis and radiocarbon dating. Radiocarbon 42:199–208
Hopkins TL, Kramer KJ (1992) Insect cuticle sclerotization. Annu Rev Entomol 37:273–302
Kornexl BE, Gehre M, Höffling R, Werner RA (1999) On-line δ18O measurement of organic and inorganic substances. Rapid Commun Mass Spectrom 13:1685–1693
Kramer KJ, Hopkins TL, Schaefer J (1995) Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem Mol Biol 25:1067–1080
Leavitt SW, Danzer SR (1993) Method for batch processing small wood samples to holocellulose for stable-carbon isotope analysis. Anal Chem 65:87–89
Leng MJ, Marshall JD (2004) Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quat Sci Rev 23:811–831
Loso MG (2009) Summer temperatures during the medieval warm period and little ice age inferred from varved proglacial lake sediments in southern Alaska. J Paleolimnol 41:117–128
Lotter AF (2003) Multi-proxy climatic reconstructions. In: MacKay A, Battarbee R, Birks HJB, Oldfield F (eds) Global change in the Holocene. Arnold Publishers, London, pp 373–383
Lotter AF, Zbinden H (1989) Late-Glacial pollen analysis, oxygen-isotope record, and radiocarbon stratigraphy from Rotsee (Lucerne), Central Swiss Plateau. Eclogae Geol Helv 82:191–202
Lotter AF, Eicher U, Siegenthaler U, Birks HJB (1992) Late-glacial climatic oscillations as recorded in Swiss lake sediments. J Quat Sci 7:187–204
Miller RF, Voss-Foucart M-F, Toussaint C, Jeuniaux C (1993) Chitin preservation in quaternary Coleoptera: preliminary results. Palaeogeogr Palaeoclimatol Palaeoecol 103:133–140
Moore JJ, Hughen KA, Miller GH, Overpeck JT (2001) Little ice age recorded in summer temperature reconstruction from varved sediments of Donard Lake, Baffin Island, Canada. J Paleolimnol 25:503–517
Muzzarelli RAA (1977) Chitin. Pergamon Press, Oxford, 309 p
Nation JL (2002) Insect physiology and biochemistry. CRC Press, Boca Raton 485 p
Pariser ER, Lombordi DP (1980) Chitin source book: a guide to research literature. Wiley, New York
Reguera G, Leschine SB (2001) Chitin degradation by cellulolytic anaerobes and facultative aerobes from soils and sediments. FEMS Microbiol Lett 204:367–374
Richards AG (1978) The chemistry of insect cuticle. In: Rockstein M (ed) Biochemistry of insects. Academic Press, New York, pp 205–232
Roberts I, Urey HC (1939) Kinetics of the exchange of oxygen between benzoic acid and water. J Am Chem Soc 61:2580–2584
Schaefer J, Kramer KJ, Garbow JR, Jacob GS, Stejskal EO, Hopkins TL, Speirs RD (1987) Aromatic cross-links in insect cuticle: detection by solid-state 13C and 15N NMR. Science 235:1200–1204
Schimmelmann A, DeNiro MJ (1985) Determination of oxygen stable isotope ratios in organic matter containing carbon, hydrogen, oxygen, and nitrogen. Anal Chem 57:2644–2646
Schimmelmann A, DeNiro MJ (1986a) Stable isotopic studies on chitin. III. The D/H and 18O/16O ratios in arthopod chitin. Geochim Cosmochim Acta 50:1485–1496
Schimmelmann A, DeNiro MJ (1986b) Stable isotopic studies on chitin, measurements on chitin/chitosan isolates and d-glucosamine hydrochloride from chitin. In: Muzzarelli RAA, Jeuniaux C, Gooday GW (eds) Chitin in nature and technology. Plenum Press, New York, pp 357–364
Schimmelmann A, DeNiro MJ, Poulicek M, Voss-Foucart M-F, Goffinet G, Jeuniaux C (1986) Stable isotopic composition of chitin from arthropods recovered in archaeological contexts as palaeoenvironmental indicators. J Archaeol Sci 13:553–566
Schwalb A (2003) Lacustrine ostracodes as stable isotope recorders of late-glacial and holocene environmental dynamics and climate. J Paleolimnol 29:265–351
Smol JP (2008) Pollution of lakes and rivers: a paleoenvironmental perspective. Blackwell Publishing, Oxford
Stankiewicz BA, Van Bergen PF, Duncan IJ, Carter JF, Briggs DEG, Evershed RP (1996) Recognition of chitin and proteins in invertebrate cuticles using analytical pyrolysis/gas chromatography and pyrolysis/gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 10:1747–1757
Stankiewicz BA, Briggs DEG, Evershed RP, Flannery MB, Wuttke M (1997a) Preservation of chitin in 25-million-year-old fossils. Science 276:1541–1543
Stankiewicz BA, Briggs DEG, Evershed RP, Duncan IJ (1997b) Chemical preservation of insect cuticle from the Pleistocene asphalt deposits of California, USA. Geochim Cosmochim Acta 61:2247–2252
Stankiewicz BA, Briggs DEG, Evershed RP (1997c) Chemical composition of paleozoic and mesozoic fossil invertebrate cuticles as revealed by pyrolysis-gas chromatography/mass spectrometry. Energy Fuels 11:515–521
Stankiewicz BA, Briggs DEG, Evershed RP, Miller RF, Bierstedt A (1998) The fate of chitin in quaternary and tertiary strata. In: Stankiewicz BA, Van Bergen PF (eds) Nitrogen-containing macromolecules in biosphere and geosphere, American Chemical Society, pp 211–224
Sturz H, Robinson J (1986) Anaerobic decomposition of chitin in freshwater sediments. In: Muzzarelli RAA, Jeuniaux C, Gooday GW (eds) Chitin in nature and technology. Plenum Press, New York, pp 531–538
Van der Kaaden A, Boon JJ, De Leeuw JW, De Lange F, Schuyl PJW, Schulten H-R, Bahr U (1984) Comparison of analytical pyrolysis techniques in the characterization of chitin. Anal Chem 56:2160–2165
Van Hardenbroek M, Heiri O, Grey J, Bodelier PLE, Verbruggen F, Lotter AF (in press) Fossil chironomid δ13C as a proxy for past methanogenic contribution to benthic food-webs in lakes? J Paleolimnol. doi 10.1007/s10933-009-9328-5
Von Grafenstein U, Erlenkeuser H, Brauer A, Jouzel J, Johnsen SJ (1999) A mid-European decadal isotope-climate record from 15,500 to 5000 years B.P. Science 284:1654–1657
Von Gunten L, Heiri O, Bigler C, Van Leeuwen J, Casty C, Lotter AF, Sturm M (2008) Seasonal temperatures for the past ~400 years reconstructed from diatom and chironomid assemblages in a high-altitude lake (Lej da la Tscheppa, Switzerland). J Paleolimnol 39:283–299
Walker IR (2001) Midges: Chironomidae and related Diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments volume 4 zoological indicators. Kluwer Academic Publishers, Dordrecht, pp 43–66
Walker IR, Cwynar LC (2006) Midges and palaeotemperature reconstruction—the North American experience. Quat Sci Rev 25:1911–1925
Wang Y, Francis DR, O’Brien DM, Wooller MJ (2008) A protocol for preparing fossil chironomid head capsules (Diptera: Chironomidae) for stable isotope analysis in paleoclimate reconstruction and considerations of contamination sources. J Paleolimnol 40:771–781
Waveren Van (1994) Chitinous palynomorphs and palynodebris representing crustacean exoskeleton remains from sediments crustaceans of the Banda Sea (Indonesia). Scripta Geol 105:1–25
Wissel H, Mayr C, Lücke A (2008) A new approach for the isolation of cellulose from aquatic plant tissue and freshwater sediments for stable isotope analysis. Org Geochem 39:1545–1561
Wolfe BB, Edwards TWD, Elgood RJ, Beuning KRM (2001) Carbon and oxygen isotope analysis of lake sediment cellulose: methods and applications. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments. Volume 2: physical and chemical techniques. Kluwer Academic Publishers, Dordrecht, pp 373–400
Wooller MJ, Francis DR, Fogel ML, Miller GH, Walker IR, Wolfe AP (2004) Quantitative paleotemperature estimates from δ18O of chironomid head capsules preserved in arctic lake sediments. J Paleolimnol 31:267–274
Wooller MJ, Wang Y, Axford Y (2008) A multiple stable isotope record of late quaternary limnological changes and chironomid paleoecology from northeastern Iceland. J Paleolimnol 40:63–77
Acknowledgments
We thank Arnold van Dijk and Gijs Nobbe for laboratory assistance. Jan van Tongeren helped with Scanning Electron Microscopy. Maarten van Hardenbroek provided fruitful discussions. We thank Matthew J. Wooller, an anonymous reviewer, and an associate editor of the Journal of Paleolimnology for valuable comments on an earlier version of the manuscript. This is publication number DW-2009-5001 of the Darwin Center for Biogeosciences, Utrecht, The Netherlands (www.darwincenter.nl), which financially supported this study. This is Netherlands Research School of Sedimentary Geology publication no. 20090701.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Verbruggen, F., Heiri, O., Reichart, GJ. et al. Effects of chemical pretreatments on δ18O measurements, chemical composition, and morphology of chironomid head capsules. J Paleolimnol 43, 857–872 (2010). https://doi.org/10.1007/s10933-009-9374-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-009-9374-z