Abstract
Oxygen isotope (δ18O) measurements on the exoskeletons of aquatic insects can be used to reconstruct changes in the δ18O of ambient water and, indirectly, to infer the climate and environmental conditions at the time of tissue synthesis. Prior to stable isotope analysis, it is often necessary to chemically pretreat insect remains to remove allochthonous organic and inorganic compounds without altering the δ18O signature. We tested the effectiveness and impact of duration of exposure to a buffered 2 M ammonium chloride (NH4Cl) solution for removing carbonates at neutral pH from chironomid head capsules, water beetle sclerites and marine crab remains prior to stable isotope analysis. Immersion in NH4Cl for 24 h efficiently removed the effect of carbonates with no long-term effects of prolonged exposure observed. Furthermore, we assessed the variability in δ18O values within and between individual sclerites (exoskeleton parts) of both modern and fossil water beetle remains. Both modern and fossil specimens had similar intra-sclerite variability in δ18O values (~ 2‰ range). In contrast, modern specimens had much smaller inter-sclerite variability (< 0.9‰ range) compared with fossil specimens from the same sample (up to 10‰ range). The high inter-sclerite variability observed in fossil material likely results from the nature of fossil material: a mix of sclerites from a 1–2–L sample bin, originating from different individuals that may have existed at different times and under different environmental conditions. We therefore recommend that material to be analysed for stable isotopes be sampled at high temporal resolution to reduce uncertainties in paleotemperature estimates derived from water beetle δ18O records.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen isotope ratios in the water of hydrologically open lakes (δ18Olakewater) dominantly reflect the isotopic composition of precipitation (δ18Oprecipitation) received by the lake via rainfall, streams and groundwater and thereby, indirectly, record a climate signal (Dansgaard 1964; Clark and Fritz 1997; Leng and Marshall 2004). The δ18O of the exoskeleton of aquatic insects has been shown to reflect the δ18Olakewater at the time of cuticle formation (Wooller et al. 2004; Verbruggen et al. 2011), with around 70% of the oxygen required for biosynthesis acquired from the host lake water itself (Wang et al. 2009; Soto et al. 2013). Highly sclerotized chitinous remains of aquatic insects are generally abundant and well-preserved within lake sediments, with δ18O values on their fossil remains having the potential to provide information on paleoclimate (Wooller et al. 2004; Verbruggen et al. 2010b; Lasher et al. 2017; van Hardenbroek et al. 2018). In lacustrine environments, stratigraphic changes in insect δ18O values can be used to infer changes in δ18Olakewater and, indirectly, past climate and environmental conditions. Biosynthesis of the chitinous cuticles does not seem to be affected by temperature-dependent fractionation processes (Schilder et al. 2015), in contrast to other lacustrine material, such as biogenic carbonate (Leng and Barker 2006). Moreover, the ability to identify insect remains under a microscope minimizes the possibility of including terrestrial contamination commonly faced by records derived for aquatic cellulose and algal lipids (Sauer et al. 2001; Leng and Barker 2006).
Chemical pretreatment of chitinous remains prior to stable isotope analysis is needed to eliminate endogenic or authigenic carbonates and allochthonous organic matter that may affect the δ18O signal. Conventional pre-treatment methods use acid (such as HCl or HF) to remove carbonates (Wooller et al. 2004; Brooks et al. 2007; Chambers et al. 2011), but at low pH oxygen can be exchanged between organic materials and water used in the pretreatment procedure (Wedeking and Hayes 1983). Moreover, the use of such acids was found to alter the chemical composition of chironomid cuticles by selective removal of chitin relative to proteins, resulting in decreased δ18O values compared to untreated controls (Verbruggen et al. 2010a). By contrast, pretreatment with a 2 M ammonium chloride (NH4Cl) solution buffered with sodium hydroxide (NaOH) can be undertaken to remove sedimentary carbonates at a circum-neutral pH (Hieltjes and Lijklema 1980) in a way that does not promote oxygen exchange or alter δ18O values of chironomid cuticles (Verbruggen et al. 2010b). During pre-treatment, the NH4Cl reacts with calcium carbonate (CaCO3), if present within the sample, to form calcium chloride (CaCl2) and ammonium carbonate ((NH4)2CO3), which rapidly degrades to gaseous ammonia (NH3) and CO2. As such, the pretreatment removes carbonate at neutral pH, minimising exchange of OH-groups between water and insect cuticle and preserving the original δ18O signal of insect cuticles (Verbruggen et al. 2010b). It remains unclear, however, how efficient NH4Cl pretreatment is in removing all residual carbonates in a sample, and whether it affects δ18O values of individual insect groups differently.
A second part of this study investigates variability of δ18O values of insect exoskeleton fragments. Fossil insect remains preserved within sediments are rarely intact specimens and instead, often comprise various parts of the exoskeleton from different individuals. It is not known how variable δ18O values of sclerite fragments from the same individual are, but this is important to consider when evaluating correlations between δ18O values of sclerite fragments and δ18O of precipitation (Gröcke et al. 2006, 2011; van Hardenbroek et al. 2013).
To address these issues, we designed a series of experiments to test (1) the efficiency of NH4Cl to remove artificially added carbonate (2) the impact of NH4Cl exposure duration on the δ18O of chironomid larvae, water beetle sclerites and a widely used purified chitin standard derived from marine crab shells; and (3) the extent to which there is natural variability in δ18O values within and between water beetle sclerites of modern and fossil material. Experiments 1 and 2 tested the effect of NH4Cl on chironomid larvae, water beetle sclerites and the chitin standard derived from marine crab shells. Chironomid larvae (Chironomus riparius) were obtained from a commercial source (Discusfarm Marsilea, Lelystad, The Netherlands). In the third and final experiment, only beetle sclerites were tested, as their large size enabled an investigation of within-sclerite variability, unlike those of chironomids.
Materials and methods
Soft tissues were left to decay in water for 3 months and remaining material was manually removed from head capsules with fine forceps. For the beetle chitin, a specimen of the genus Dytiscus was extracted from the surface sediments of Lake Nimetön (61.23°N, 25.19°E, Finland). The elytron was ground into a fine powder using an agate pestle and mortar to homogenize the sample. As a third sample, we used a marine crab chitin standard, which was purified by the supplier (Sigma-Aldrich, St. Louis, MO) using the method of Skujins et al. (1965).
For experiment 3, which tested intra- and inter-sclerite variability in δ18O values, we analysed modern and fossil specimens of North American water beetles from the genera Dytiscus, Hydroporus and Helophorus. Adults and their larvae of these three genera are fully aquatic, with Dytiscus and Hydroporus (predaceous diving beetles) inhabiting the pelagic zone of ponds and shallow lakes, whilst intermittently diving down deeper into the water column to catch their prey (Larson et al. 2000). Adults of the water scavenger beetle Helophorus inhabit the benthic zone of ponds and shallow lakes, and their larvae are known to be predaceous (Angus 1973; Landin 1980). In North America, the larvae of these three genera typically emerge within 6–8 days in spring or summer; Hydroporus in late July to early August (Larson et al. 2000), whereas Helophorus and Dytiscus species typically emerge weeks to months earlier in the year (Smetana 1988; Hilsenhoff 1995). All of the beetle specimens analysed in this study are fully aquatic and were collected from perennially wet ponds, lakes or associated organic deposits representing tundra lakes, peatland and/or mire sediments. The sample material consisted of heads, pronota, elytra, metasterna, hind legs and abdomens (Figs. 3 and 4). For details of collection location and age of the modern and fossil beetle specimens, see Electronic Supplementary Material (ESM) Tables 1 and 2.
Experimental design
Experiment 1 tested the effectiveness of a buffered 2 M NH4Cl solution in removing artificially added carbonate. All samples, including controls, were immersed in 10% KOH for 2 h at room temperature as an initial cleaning step to remove any remaining soft tissues, following Verbruggen et al. (2010b), and rinsed with demineralized water over a 41-μm-mesh sieve. Next, 1 g of calcium carbonate (CaCO3) powder was added to 1 mg of chironomid, beetle and crab sample batches. These samples were then soaked in 5 ml of a 2 M NH4Cl solution buffered with NaOH at a pH of 7.5 (Verbruggen et al. 2010a) for either 2 h or 24 h. All samples were rinsed with deionised water and freeze-dried before δ18O analysis. A subset of each CaCO3-treated batch received no pretreatment with NH4Cl and acted as a positive control. Measured δ18O values of treated samples were compared with those obtained for untreated samples, which received no CaCO3 or pretreatment with NH4Cl.
Experiment 2 tested whether the duration of exposure to NH4Cl had an effect on the δ18O of aquatic insect remains. Batches of samples were drawn after 2, 12, 24 and 48 h of exposure to 5 ml of the buffered NH4Cl solution at room temperature and rinsed with deionised water. Initial batches of each group were measured as an untreated control group receiving no pretreatment with NH4Cl.
We tested for significant differences between mean δ18O values of control samples and the mean δ18O values for each experiment using the non-parametric Kruskal–Wallis test and between treatments in each experiment with Mann–Whitney tests (Past software version 2.14, Hammer et al. 2001).
Analytical methods
All samples except the three modern Dytiscus beetle specimens were analysed for 18O/16O in the Stable Isotope Biogeochemistry Laboratory at Durham University. Oxygen isotope ratios were determined using a High Temperature Conversion Elemental Analyzer (Thermo Finnigan TC/EA) coupled to a continuous flow Delta V Advantage isotope ratio mass spectrometer (IRMS) (Thermo Scientific) with no-helium dilution aspect in the ConFlo III, because of the small sample weights obtained for chitin samples. Results were calibrated against three international reference standards (IAEA-600, -601, -602) and two in-house standards (TU-2 and C9752). Data filtering followed a strict protocol to assess the quality of the electrical signal retrieved for samples, including reference standards, on the mass spectrometer. In total, three measurements of standards were removed from the analysis because of their likely low weight and/or incomplete combustion. Replicate measurements of standards indicated a mean analytical precision of 0.4‰ (1σ) for batch 1 and 0.3‰ (1σ) for batch 2 (ESM Table 3).
Modern Dytiscus beetle specimens were analysed in the Stable Isotope Biogeochemistry Laboratory at McMaster University on a Costech TC/EA connected to a Thermo Finnigan Delta Plus XP IRMS without helium dilution. The results were calibrated against in-house chitin and benzoic acid reference standards, and ANU sucrose as international reference material (ESM Table 3). Repeated measurement of standards indicated a mean analytical precision of < 0.4‰ (1σ).
Results
Effect of NH4Cl pretreatment on invertebrate δ18O
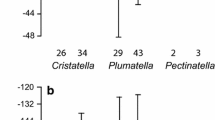
Experiment 1 tested the efficiency of NH4Cl pretreatment for removing residual carbonates from chironomid, beetle and marine crab chitin prior to stable isotope analysis. The artificial addition of CaCO3 was found to significantly change the δ18O of chironomid and beetle samples only compared to controls (Fig. 1), whereas the CaCO3 and crab chitin samples had similar δ18O values (results of Mann–Whitney tests in ESM Tables 4b and 4c). After 2 h and 24 h of treatment with NH4Cl, the δ18O values of the aquatic insect samples did not indicate an effect of CaCO3 anymore, as the samples were not statistically different from the untreated controls (ESM Tables 4b and 4c).
Δ18O values (δ18O untreated–treated) for a chironomid, b beetle and c crab chitin samples, having undergone pretreatment experiments (filled symbols), and untreated control samples (open symbols). Pretreatment experiments included the artificial addition of CaCO3 with no NH4Cl exposure (carbonate), artificial addition of CaCO3 with either 2 h exposure in NH4Cl (2 h NH4Cl) or 24 h exposure in NH4Cl (24 h NH4Cl). Pretreatment experiments found to be statistically different from untreated control samples are indicated (*)
Experiment 2 tested the impact of prolonged exposure to NH4Cl on chironomid, beetle and crab δ18O (Fig. 2). One outlier in the chironomid batch exposed to NH4Cl for 24 h, possibly the result from contamination by CaCO3 used in experiment 1, was excluded from further statistical analyses. No significant differences in δ18O values were found for samples exposed to NH4Cl for 2 h and those obtained for samples exposed for 12, 24 or 48 h (H > 2.6, p > 0.2, ESM Table 4a). A significant difference was found, however, for untreated crab controls and those exposed to NH4Cl for any duration, possibly resulting from removing calcium carbonate that is an integrated part of crustacean exoskeletons (Vincent 2002; Gupta 2011). The effect of removing the internal carbonate on δ18O was significant when comparing control and exposure to NH4Cl for 2 h, but no further change in δ18O was found upon prolonged exposure to NH4Cl (ESM Table 4e).
Intra- and inter-sclerite variability in beetle δ18O
We investigated the extent of intra- and inter-sclerite variability in stable oxygen isotope values in both modern and fossil water beetles from North America. Within the exoskeletons of three modern Dytiscus harrisii diving beetles, we observed a variability in δ18O values (standard deviation) of 0.7, 0.4, and 0.6‰, with a range of ~ 2‰ in any body part (Fig. 3a–c). Moreover, a difference in mean δ18O values of 0.3‰ was observed between the left and the right elytron. For the specimen presented in Fig. 3a, some of the ventral sclerites were also measured. The abdomen and hind legs displayed a standard deviation of 0.7‰ and 0.4‰, respectively.
The intra-sclerite variability in δ18O values within intact fossil remains of elytra from the genus Hydroporus are presented in Fig. 3d–g. On average for the four fossil specimens analysed, δ18O values were 1.05‰ higher for the humeral (top) section of the elytron compared to the apical (base) section. The intra-sclerite variability of fossil material was similar to that of modern material (ranges of ~ 2‰ in the fossil elytra), suggesting that fossil elytra contain a similar stable isotope ‘signal’ as modern elytra. Modern beetle specimens were more than 10 × larger and sampled in more detail than fossil specimens, limiting the comparison of intra-sclerite variability in modern and fossil material.
Standard deviations of 0.2 to 0.5‰ were observed for δ18O values of individual sclerites of the modern Dytiscus harrisii specimens (e.g. head, elytron, pronotum, abdomen), with the elytra displaying the smallest standard deviation in δ18O values compared with other body parts (Fig. 4a). By contrast, high inter-sclerite variability was observed for fossil specimens (Fig. 4b). Sclerites obtained from fossil assemblages of 1–2–L samples of the same age and collection location displayed standard deviations of 0.1 to 3.2‰ (total range up to 10‰).
Variation in δ18O values for individual sclerites originating from both modern (museum) and fossil specimens of water beetles from North America. Fossil specimens from the same 1–2–L sample bin originate from the same site and same age fossil insect assemblage. All fossil specimens originated from a different individual sclerite fragment. Error bars indicate variability within a single body part (intra-sclerite variability). See ESM Tables 1 and 2 for details on the collection location and age of modern and fossil beetle specimens, respectively
Discussion
Effect of NH4Cl pretreatment on invertebrate δ18O
We confirm the findings of Verbruggen et al. (2010b) for chironomids and further demonstrate that duration of exposure to NH4Cl also has no significant effect on the δ18O of water beetles and a commonly used purified chitin standard derived from marine crab shells. Combined, the two experiments presented here indicate that, within the 0.4‰ analytical error of our measurements, a 24–48 h exposure to a buffered NH4Cl solution can effectively remove all carbonates from aquatic insect remains prior to stable isotope analysis, without affecting the δ18O values of the sample material. The buffered NH4Cl method was originally designed to measure carbonate-bound phosphorus in sediments and further developed by Hieltjes and Lijklema (1980) for lake sediments. Hieltjes and Lijklema (1980) used 50 ml of 1 M NH4Cl solution buffered to pH 7 and 50 mg of dry sediment from Lake Brielle, The Netherlands, containing between 8.5 and 18.2% calcium carbonate by wt. They observed nearly complete carbonate removal (93.5 ± 6.7%, average ± standard deviation) after 2 h of exposure to 1 M NH4Cl, which is half as concentrated as the 2 M NH4Cl solution used in our study and by Verbruggen et al. (2010b). No study to date has specifically tested the removal efficiency of the NH4Cl method on different types of sedimentary and authigenic carbonates (e.g. calcite, aragonite, dolomite), but based on Hieltjes and Lijklema (1980) and Verbruggen et al. (2010b) and the current study, a buffered 2 M NH4Cl will effectively remove carbonates from soils and lake sediments, especially when left to react for 24 h, which is 12 times as long as in the study by Hieltjes and Lijklema (1980).
Intra- and inter-sclerite variability in beetle δ18O
Intra-sclerite variability (~ 2‰ range) in δ18O values for both modern and fossil water beetle sclerites (Fig. 3) was relatively low compared to the high inter-sclerite variability observed between fossil specimens originating from the same sample (up to 10‰ range; Fig. 4b). In general, the modern elytra displayed the most consistent δ18O values compared with other sclerites, but variability in δ18O does not seem to be systematic by region or body part, based on the sampling locations and specimens analysed here (Fig. 3). This small within-sclerite variability could result from remaining soft tissues (e.g. inside the cavities of legs and heads) with different δ18O values, thereby altering the δ18O signature within sclerites originating from the same individual.
As there is also relatively low inter-sclerite variability in δ18O values for modern beetle specimens (< 0.9‰ range; Fig. 4a), the high inter-sclerite variability observed in fossil material (up to 10‰ range) likely reflects either real differences in recorded source-water isotopes or interspecies differences in vital effects and/or biological fractionation. Typically, the paleoecological analysis of fossil beetle assemblages requires between 1 and 2 L of organic sediment to yield sufficient material to constrain paleoenvironmental interpretations (Elias 2001). The resulting fossil assemblages can therefore span thousands of years during which large shifts in the δ18O of precipitation and lake water δ18O are likely to have occurred. Furthermore, specimens retrieved from fossil beetle assemblages are often composed of a mix of sclerites from different individuals and/or species within the genera of interest, each with slightly different diet, habitat preferences, metabolism or utilisation of body water (deBruyn and Ring 1999). Differences in the seasonality of growth and/or scleral synthesis within and between the individuals and species present within the fossil assemblage could also explain some, or even most, of the observed variability in beetle δ18O values. No studies have investigated the seasonal variability of δ18O values in water beetle sclerites, but it is thought that aquatic beetle species mostly synthesize chitin during and shortly before emergence. For the three genera in this study, the larvae emergence typically takes place within a week in late spring or summer; Hydroporus in late July to early August (Larson et al. 2000), whereas Helophorus and Dytiscus species typically emerge weeks to months earlier in the year, usually in spring or early summer (Smetana 1988; Hilsenhoff 1995). Generally, emergence happens close to the warmest month, especially at higher latitudes (David Larson pers. commun.). Previous work comparing δ18O values of modern water beetles in North America and monthly δ18O values of modelled precipitation, found the strongest correlations for July and August (van Hardenbroek et al. 2013), also suggesting that variability of δ18O values in water beetle sclerites is limited to variability of lake water δ18O values during the warmest months.
The low intra-sclerite and high inter-sclerite variability in δ18O from fossil beetle specimens has important implications for reconstructing paleoclimate. If it is assumed that the δ18O of environmental waters varies with temperature at a slope of 0.7‰ per °C (Dansgaard 1964), the mean observed variability in δ18O of fossil specimens derived from the same fossil assemblage (~ 1.5‰) corresponds to an uncertainty in paleotemperature estimate of ± 2.6 °C. Analysis of multiple beetle specimens from the same stratigraphic interval or assemblage will thus provide a larger, but more realistic range of beetle δ18O values and may give valuable information on short-term variability (i.e. within the time period represented by the fossil sample) in lake water isotope composition, as has been demonstrated with ostracod valves (Escobar et al. 2010).
In an attempt to test its utility in paleoenvironmental analysis, Motz (2000) measured stratigraphic changes in δ18O values of fossil bark beetle fragments and other tree-feeding insects from ca. 18,000-year-old sediment at the Gardena site, Illinois, and compared them to measured δ18Ocellulose values from the same horizons. High variability (standard deviation 0.62 to 1.76‰) was observed for fossil bark beetle fragments analysed from the same stratigraphic interval, likely because of (1) real differences in the fractionation of stable oxygen isotopes in different (parts of) trees as a result of differential evapotranspiration and/or (2) the large sample volumes (28–34 kg) used, limiting the temporal resolution possible for the fossil insect δ18O record. It would be expected that analysing material sampled at higher temporal resolution would reduce the number of sclerites in a sample, and thus the inter-sclerite variability. For example, high-resolution aquatic insect δ18O records have been produced using chironomid head capsules, which are generally more abundant in lake sediments than remains of water beetles (Verbruggen et al. 2010b; Arppe et al. 2017; Lombino 2014; Lasher et al. 2017).
Conclusions
We demonstrated that pretreatment with buffered NH4Cl efficiently removes the effect of artificially added calcium carbonate on the δ18O values of chironomid and beetle chitin, without affecting their δ18O measure, even after prolonged (48 h) exposure. Moreover, we assessed the extent to which there is internal and within-sample variability in δ18O of modern and fossil specimens of water beetles. The variability in δ18O values within sclerites (exoskeleton parts) of modern specimens was similar to that observed for fossil specimens. Standard deviations of δ18O values between individual modern water beetles was only 0.2 to 0.5‰, and more than six times greater between individual fossil water beetle remains (0.1 to 3.2‰). Most likely, the large variability in fossil samples is caused by the low temporal resolution of the samples studied here, which span thousands of years. We therefore recommend that sediment records be sampled at higher temporal resolution for stable isotope studies of beetles, instead of large-volume sampling, which is often necessary for the paleoecological study of beetle assemblages.
References
Angus RB (1973) The habitats, life histories and immature stages of Helophorus F. (Coleoptera: Hydrophilidae). Trans R Entomol Soc Lond 125:1–26
Arppe L, Kurki E, Wooller MJ, Luoto TP, Zajączkowski M, Ojala AEK (2017) A 5500-year oxygen isotope record of high arctic environmental change from southern Spitsbergen. Holocene 27:1948–1962
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of palaearctic Chironomidae larvae in paleoecology. Quat Res Tech Guide 10:1–276
Chambers FM, van Geel B, van der Linden M (2011) Considerations for the preparation of peat samples for palynology and for the counting of pollen and non-pollen palynomorphs. Mires Peat 7:1–14
Clark I, Fritz I (1997) Environmental isotopes in hydrogeology. Lewis, Boca Raton
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
deBruyn AMH, Ring RA (1999) Comparative ecology of two species of Hydroporus (Coleoptera: Dytiscidae) in a high Arctic oasis. Can Entomol 13:405–420
Elias SA (2001) Coleoptera and trichoptera. In: Smol JP, Last WM (eds) Tracking environmental change using lake sediments. Springer, Dordrecht pp, pp 67–80
Escobar J, Curtis JH, Brenner M, Hodell DA, Holmes JA (2010) Isotope measurements of single ostracod valves and gastropod shells for climate reconstruction: evaluation of within-sample variability and determination of optimum sample size. J Paleolimnol 43:921–938
Gröcke DR, Schimmelmann A, Elias S, Miller RF (2006) Stable hydrogen-isotope ratios in beetle chitin: preliminary European data and re-interpretation of North American data. Quat Sci Rev 25:1850–1864
Gröcke DR, van Hardenbroek M, Sauer PE, Elias SA (2011) Hydrogen isotopes in beetle chitin. In: Gupta N (ed) Chitin, topics in geobiology, vol 34. Springer, Dordrecht, pp 105–116
Gupta NS (2011) Transformation of chitinous tissues in elevated pressure–temperature conditions: additional insights from experiments on plant tissues. In: Gupta NS (ed) Chitin, formation and diagenesis. Springer, Dordrecht, pp 153–168
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:9
Hieltjes AHM, Lijklema L (1980) Fractionation of inorganic phosphates in calcareous sediments. J Environ Qual 9:405–407
Hilsenhoff WL (1995) Dytiscidae and Noteridae of Wisconsin (Coleoptera). V. Distribution, habitat, life cycle and identification of species of Hydroporinae, except Hydroporus Clairville sensu lato. Great Lakes Entomol 26:1–23
Landin J (1980) Habitats, life histories, migration and dispersal by flight of two water beetles Helophorous brevipalpis and H. strigifrons (Hydrophilidae). Ecography 3:190–201
Larson DJ, Alarie Y, Roughley RE (2000) Predaceous diving beetles (Coleoptera: Dytiscidae) of the Nearctic region, with emphasis on the fauna of Canada and Alaska. NRC Research Press, Ottawa
Lasher GE, Axford Y, McFarlin JM, Kelly MA, Osterberg EC, Berkelhammer MB (2017) Holocene temperatures and isotopes of precipitation in Northwest Greenland recorded in lacustrine organic materials. Quat Sci Rev 170:45–55
Leng MJ, Barker PA (2006) A review of the oxygen isotope composition of lacustrine diatom silica for paleoclimate reconstruction. Earth Sci Rev 75:5–27
Leng MJ, Marshall JD (2004) Paleoclimate interpretation of stable isotope data from lake sediment archives. Quat Sci Rev 23:811–831
Lombino A (2014) The systematics of oxygen isotopes in chironomids (Insecta: Diptera): a tool for reconstructing past climate. Ph.D. thesis, University College, London
Motz JE (2000) Oxygen and hydrogen isotopes in fossil insect chitin as paleoenvironmental indicators. Ph.D. thesis, University of Waterloo, Canada
Sauer PE, Eglinton TI, Hayes JM, Schimmelmann A, Sessions AL (2001) Compound-specific D/H ratios of lipid biomarkers from sediments as a proxy for environmental and climatic conditions. Geochim Cosmochim Acta 65:213–222
Schilder J, Tellenbach C, Möst M, Spaak P, van Hardenbroek M, Wooller MJ, Heiri O (2015) The stable isotopic composition of Daphnia ephippia reflects changes in δ13C and δ18O values of food and water. Biogeosciences 12:3819–3830
Skujins JJ, Potgieter HJ, Alexander M (1965) Dissolution of fungal cell walls by a streptomycete chitinase and β-(1 → 3) glucanase. Arch Biochem Biophys 111:358–364
Smetana A (1988) Review of the family Hydrophilidae of Canada and Alaska (Coleoptera). Mem Entomol Soc Can 120:3–316
Soto DX, Wassenaar LI, Hobson KA (2013) Stable hydrogen and oxygen isotopes in aquatic food webs are tracers of diet and provenance. Funct Ecol 27:535–543
van Hardenbroek M, Gröcke DR, Sauer PE, Elias SA (2013) North American transect of stable hydrogen and oxygen isotopes in water beetles from a museum collection. J Paleolimnol 48:461–470
van Hardenbroek M, Chakraborty A, Davies K, Harding P, Heiri O, Henderson ACG, Holmes JA, Lasher GE, Leng MJ, Panizzo VN, Roberts L, Schilder J, Trueman CN, Wooller MJ (2018) The stable isotope composition of organic and inorganic fossils in lake sediment records: current understanding, challenges, and future directions. Quat Sci Rev 196:154–176
Verbruggen F, Heiri O, Reichart GJ, de Leeuw JW, Nierop KGJ, Lotter AF (2010a) Effects of chemical pretreatments on δ18O measurements, chemical composition and morphology of chironomid head capsules. J Paleolimnol 43:857–872
Verbruggen F, Heiri O, Reichart GJ, Lotter AF (2010b) Chironomid δ18O as a proxy for past lake water δ18O: a lateglacial record from Rotsee (Switzerland). Quat Sci Rev 29:2271–2279
Verbruggen F, Heiri O, Reichart GJ, Blaga C, Lotter AF (2011) Stable oxygen isotopes in chironomid and cladoceran remains as indicators for lake-water δ18O. Limnol Oceanogr 56:2071–2079
Vincent JFV (2002) Arthropod cuticle: a natural composite shell system. Compos Part A Appl Sci 33:1311–1315
Wang YV, O’Brien DM, Jenson J, Francis D, Wooller MJ (2009) The influence of diet and water on the stable oxygen and hydrogen isotope composition of Chironomidae (Diptera) with paleoecological implications. Oecologia 160:225–233
Wedeking KW, Hayes JM (1983) Exchange of oxygen isotopes between water and organic material. Isot Geosci 1:357–370
Wooller MJ, Francis D, Fogel ML, Miller GH, Walker IR, Wolfe AP (2004) Quantitative paleotemperature estimates from δ18O chironomid head capsules preserved in Arctic lake sediments. J Paleolimnol 31:267–274
Acknowledgements
We thank Dr Pat Bouchard and Mr Anthony E. Davies at the Canadian National Collection of Insects, Agriculture and Agri-Food, Canada, for providing the modern beetle specimens for this study. We thank two anonymous reviewers and Prof. Oliver Heiri for their time and constructive feedback on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Clarke, C.L., Gröcke, D.R., Elias, S. et al. Effects of chemical pretreatment and intra- and inter-specimen variability on δ18O of aquatic insect remains. J Paleolimnol 62, 195–204 (2019). https://doi.org/10.1007/s10933-019-00085-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-019-00085-1