Abstract
Malate is an important material to various industrials and clinical applications. Bacillus subtilis is a widely used biocatalyst tool for chemical production. However, the specific enzymatic properties of malate dehydrogenase from Bacillus subtilis (BsMDH) remain largely unknown. In the present study, BsMDH was cloned, recombinantly expressed and purified to test its enzymatic properties. The molecular weight of single unit of BsMDH was 34,869.7 Da. Matrix-Assisted Laser-Desorption Ionization-Time-of-Flight Mass Spectrometry and gel filtration analysis indicated that the recombinant BsMDH could form dimers. The kcat/Km values of oxaloacetate and NADH were higher than those of malate and NAD+, respectively, indicating a better catalysis in the direction of malate synthesis than the reverse. Furthermore, six BsMDH mutants were constructed with the substitution of amino acids at the coenzyme binding site. Among them, BsMDH-T7 showed a greatly higher affinity and catalysis efficiency to NADPH than NADH with the degree of alteration of 2039, suggesting the shift of the coenzyme dependence from NADH to NADPH. In addition, BsMDH-T7 showed a relatively lower Km value, but a higher kcat and kcat/Km than NADPH-dependent MDHs from Thermus flavus and Corynebacterium glutamicum. Overall, these results indicated that BsMDH and BsMDH-T7 mutant might be promising enzymes for malate production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malate dehydrogenase (MDH, EC 1.1.1.37) is an important enzyme in organisms, mainly catalyzing the interconversion of malate and oxaloacetate (OAA) [1], which is a rate-limiting step in the tricarboxylic acid cycle (TCA). In addition, MDH plays essential roles in diverse metabolic pathways, including gluconeogenesis, lipogenesis, protection against oxidative stress, and substrate channeling [2,3,4,5]. Moreover, MDH was developed as a biomarker for molecular breeding [6], and a potential diagnosed antigen for vaccine development [7].
In various species, MDHs with different amino acid sequences and secondary structures have been reported, displaying diverse enzymatic efficiency and affinity to substrates. Generally, MDH shows a highly conserved 3-dimensional structure, existing in the forms of dimer or tetramer. MDHs from most Gram-negative bacteria and all eukaryotes are dimeric with the molecular weights ranging from 30 to 38 kDa for each subunit. In most Gram-positive bacteria and archaea, MDHs form tetramers (130–172 kDa) [5]. According to coenzyme specificity, MDHs are divided into NAD+-dependent (EC 1.1.1.37) and NADP+-dependent types (EC 1.1.1.82). Almost all MDHs from bacteria, eukaryotic cytoplasm and mitochondria are NAD+-dependent, while those from eukaryotic chloroplast are NADP+-dependent [5].
Malate is a widely used chemical in cosmetic, textile and food industries, rapid clinical diagnostic assays (aspartate aminotransferase activity and serum bicarbonate level), and the bioremediation of heavy metal pollution [8, 9]. One efficient way to produce malate is microbial fermentation, during which MDH is the key factor determining the production efficacy and cost. For example, Aspergillus flavus is a well-known malate producer. It can ferment glucose to malate with a relatively high yield, but the by-product aflatoxin needs additional costs to remove [10]. The transgenic Escherichia coli strain with restructured malic enzymes could produce 1.41 mol of malate based on 1 mol of glucose, and accumulate 34 g/L malate in the culture media [11]. The transgenic Bacillus subtilis strain with phosphoenolpyruvate carboxylase and malate dehydrogenase from Saccharomyces cerevisiae further increased malate production [12]. Although industrial production of malate using MDH activity-enhanced bacteria have been realized, further exploration of MDH from other species may provide more choices for industrial production of malate.
Bacillus subtilis is a promising biocatalyst platform, due to its well-characterized genetic background, mature fermentative technology, and high tolerance to solvent and environmental stresses owing to its thicker cell wall compared with other microorganisms [13,14,15,16,17]. In addition, B. subtilis expresses various enzymes including proteases, cellulases, xylanases and amylases. Thus, it can utilize a wide range of carbon and energy sources. Thus, based on some cheaper substrates, B. subtilis may be used to produce malate [18,19,20]. However, up to date, the specific enzyme properties of B. subtilis MDH (BsMDH) has not yet been reported, which are important basic information for further development of B. subtilis as a malate production tool. In addition, all bacterial MDHs require NAD+ as the coenzyme. Thus, the yield of malate partially relies on the speed of NAD+ regeneration. To resolve this question, one strategy is the enhancement of coenzyme regeneration mechanism. For example, Lu and Mei [21] enhanced coenzyme formation mechanisms in E. coli, which effectively elevated the overall efficacy of NADP+-dependent enzymes. Alternatively, switch of the coenzyme specificity from insufficient coenzyme to abundant coenzyme by mutation of the coenzyme binding sites might also increase the final efficacy of coenzyme-dependent enzymes. Following this thought, mutation of the NAD+-binding site in BsMDH to NADP+-binding sequence might create novel NADP+-dependent bacterial MDHs, which might contribute more selections for industrial applications.
In this study, we cloned, recombinantly expressed and purified BsMDH. Next, its enzymatic properties were characterized. Moreover, to create more MDHs with different properties, the coenzyme binding sites of BsMDH were mutated and their specificity to coenzymes were tested. Overall, these results would contribute more MDH protein sequences for industrial applications and also promote our understanding of MDH functions in B. subtilis.
2 Materials and Methods
2.1 Gene Cloning
Bacillus subtilis ATCC 6051 was purchased from the China General Microbiological Culture Collection Center (CGMCC). Genomic DNA was extracted using a bacterial genomic DNA rapid extraction kit (Protein Biotech, Beijing, China). The BsMDH gene (GenBank accession number NC020507.1) was amplified using 2×Pfu PCR Master Mix (Protein Biotech, Beijing, China) with primers, 5′-AAAATGTGCATATGATGGGAAATACTCGT-3′ and 5′-AGCCGCTCGAGTTAGGATAATACTTTCA-3′. Next, the PCR product was ligated to the pET- 28b(+) plasmid using the restriction enzymes Nde I and Xho I (New England BioLabs Inc., UK). The vector was transformed into E. coli DH5α. After DNA sequencing, the correct recombinant plasmid was extracted.
MEGA 7.0 was used to construct the phylogenetic tree of BsMDH and other MDHs with 1000 bootstraps [22]. Sequence alignment was conducted using Clustal W [23]. Prediction of secondary structure was conducted using the PSIPRED 4.0 tool online (http://bioinf.cs.ucl.ac.uk/psipred/).
2.2 Protein Expression and Purification
The recombinant plasmid was transformed into E. coli Rosetta BL21(DE3) pLysS and a positive clone was cultured overnight at 37 °C in LB medium containing 30 mg/L kanamycin (Macklin, Shanghai, China) and 30 mg/L chloramphenicol (Macklin, Shanghai, China). Next, E. coli was inoculated into fresh LB medium. When the OD600nm reached 0.4–0.6, 0.5 mM IPTG (Macklin, Shanghai, China) was added and then further cultured at 20 °C for 20 h. E. coli cells were harvested by centrifugation at 5000 rpm for 20 min, and then suspended in 20 mL of LEW buffer (50 mM NaH2PO4, 300 mM NaCl, pH 8.0). Cells were homogenized by ultrasonication for 40 min on ice (20 s for sonication and 40 s for cooling, 40 cycles). After centrifugation at 10,000 rpm and 4 °C for 20 min, his6-tagged target proteins in the supernatant were purified using the Co2+-affinity chromatography (Genscript, Nanjing, China) at 4 °C. The obtained protein was visualized by the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE).
2.3 Molecular Mass Determination
Protein concentration was determined using a Quick Start Bradford protein assay kit (Bio-Rad, Irvine, USA) with bovine serum albumin (BSA) as the standard, and approximately 2.5 mg of BsMDH in total was analyzed using gel filtration for preliminary molecular calculation. After treatment with thrombin (Macklin, Shanghai, China), the BsMDH without his6-tag was visualized on native PAGE, and its molecular weight was determined using a Matrix-Assisted Laser-Desorption Ionization-Time-of-Flight Mass Spectrometry (MALDI-TOF MS) 5800 Analyzer (AB SCIEX, USA) as previously described [24]. Briefly, 0.6 µl of sample was mixed directly onto the target plate with the matrix solution (1:3 vol/vol), air-dried and then measured usign the machine. The mass spectrometry data have been deposited to the figshare (https://doi.org/10.6084/m9.figshare.13525388). Moreover, a Gel Permeation Chromatography coupled to Multi-Angle Light Scattering (GPC-MALS, Wyatt Technology Co., USA) system was also employed to detect the molecular weight of BsMDH.
2.4 Determination of Enzyme Activity and Kinetic Properties
The enzymatic properties of the recombinantly expressed protein were determined as follows. MDH catalyzes the reversible transformation from oxaloacetate (OAA) and NAD(P)H to malate and NAD(P)+. Since NAD(P)H has a characteristic absorption at 340 nm, the enzyme activity can be calculated by monitoring the change of OD340 nm. The reaction system contained 100 mM Tris–HCl (pH 8.0), 2.25 mM OAA and 0.25 mM NAD(P)H. The standard reaction solution was thoroughly mixed with the appropriate amount of enzyme solution and then maintained at 25 °C for 60 s. The reduction of NAD(P)H per unit time was measured by monitoring the absorbance at 340 nm using a Cary 300 Bio UV–Visible spectrophotometer (Varian, CA, USA). Enzyme activity was expressed as micromoles of NADH oxidized/NAD+ reduced per minute of reaction time per milliliter of enzyme solution [25]. Substrate kinetics and coenzyme kinetics, including Km values for oxaloacetate, l-malate, NADH, and NADPH were determined. Km, kcat and kcat/Km were estimated based on the Michaelis–Menten plots. All experiments were conducted in triplicates independently.
2.5 Effects of pH and Temperature on Enzyme Activity
To determine the effects of pH and temperature on the enzymatic activity of BsMDH, the pH of the reaction solution was adjusted from 7.0 to 9.0 at the interval of 0.5, and the temperature was adjusted from 20 to 55 °C at the interval of 5 °C. The BsMDH activity was assessed as described above. To investigate the thermal stability of BsMDH, enzyme solution was incubated at different temperatures (from 30 to 55 °C at the interval of 5 °C) for 20 min, placed in an ice bath for 5 min and then the enzyme activities were determined at 25 °C.
2.6 Effects of Metal Ions and Compounds on Enzyme Activity
The monovalent metal ions (K+, Na+, Rb+ and Li+) and divalent ions (Mn2+, Mg2+, Co2+, Ca2+, Zn2+, Cu2+ and Ni2+) at a final concentration of 2 mM were added into the reaction system according to methods described previously [26], and then BsMDH activity was measured as described above. Similarly, the effects of three kinds of purine nucleotides (adenosine triphosphate, adenosine diphosphate and Adenosine monophosphate, abbreviated as ATP, ADP and AMP) and three kinds of metabolic compounds (α-ketoglutarate, dithiothreitol, and ethylene diamine tetraacetic acid, abbreviated as α-KG, DTT and EDTA) on BsMDH activity were also analyzed. Considering as the wide application in buffer systems, the effects of DTT and EDTA were tested at two concentrations.
2.7 Construction and Expression of BsMDH Mutants
According to the known coenzyme binding site of NADP+-dependent chMDH, the NAD+ coenzyme binding site (D36IPQLEN42) in BsMDH was replaced with the amino acid sequence of NADP+ coenzyme binding site (GSERSFQ) from chMDH [27], including the following six BsMDH mutants: BsMDH-T1a (S37), BsMDH-T1b (R39), BsMDH-T3(G36S37S40), pBsMDH-T4(G36S37R39S40), pBsMDH-T5 (G36S37R39S40Q42), pBsMDH-T7 (G36S37E38R39S40F41Q42). The primers, plasmids and strains used for these mutations are listed in Supplementary Table S1. The mutants were constructed using overlap PCR, and then ligated to pET-28b(+) plasmid by double restriction endonuclease digestion, which were further transformed in the E. coli Rosetta BL21(DE3) pLysS. After validation by Sanger sequencing, a positive clone was subjected for protein expression and purification as described above. In order to further verify whether the mutations on the coenzyme binding sites changed the secondary structure of MDH proteins, Circular Dichroism assays of wild-type and mutated MDHs were performed using a Circular Dichroism spectrometer (Tokyo, Japan). Finally, the kinetic parameters of mutated enzymes were determined using the methods described above.
3 Results
3.1 BsMDH Gene Sequence and Protein Expression
The full length BsMDH was 939 bp, encoding 312 amino acid residues. SDS–PAGE revealed a clear band at approximately 35 kDa (Fig. 1A). The result of gel filtration chromatography showed that the molecular weight of the target protein was approximately 74 kDa (Fig. 1B). After removing the his6-tag, the native PAGE image showed two bands (Supplementary Fig. S1). The MALDI-TOF MS results showed two peaks with molecular masses of 68,988.36 and 34,532.4 Da (Fig. 1C), suggesting that BsMDH in the solution might form homodimers (69.0 kDa) with the molecular weight of each subunit of 34.5 kDa. The GPC-MALS analysis showed the molecular weight of the main peak (peak 2, 93.0%) was 123.1 ± 1.653% kDa (Fig. 1D), suggesting that the forms of BsMDH in the solution of GPC-MALS might be a mixture of homodimers and homotetramers. Overall, these data suggested that BsMDH might form dimers or tetramers in different buffer systems.
Enzymatic properties of the recombinantly expressed BsMDH. A SDS–PAGE analysis. M: protein molecular weight marker. Lane 1: crude extracts of pET-28b(+) cells. Lane 2: crude extracts of cells transformed with BsMDH. Lane 3: purified BsMDH. B Gel filtration chromatography analysis. The standard curve shows the logarithmic molecular weight against elution volume. BsMDH was represented by the red circle. C MALDI-TOF-MS analysis of BsMDH without his6-tag. Two single-charged molecular ions were observed at 34532.2789, and 68988.2656 Da. BSA was used as the external standard. R means resolution, and the S means the signal to noise ratio. D Gel Permeation Chromatography coupled to Multi-Angle Light Scattering (GPC-MALS) analysis of the oligomerization state of BsMDH without his6-tag. LS (red): light scattering detector. UV (green): ultraviolet detector. dRI (blue): differential refractive index detector. Peak 2 at retention time from 20.105 min to 23.497 min was the major peak (93%), displaying Mw of 123.1 (± 1.653%) kDa, and Mz of 133.0 (± 4.023%) kDa (Color figure online)
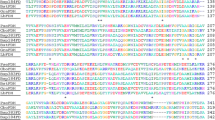
The secondary structure of BsMDH was predicted to contain 13 strands, 11 helixes and 23 coils (Fig. 2A). The phylogenetic analysis revealed that BsMDH was clustered with MDHs from Gram-positive and Gram-negative bacteria but isolated from eukaryotes (Fig. 2B). Alignment of protein sequences revealed that the NAD+ coenzyme binding site (D36IPQLEN42, Fig. 2C with black underline) was localized to a relatively less conservative area.
Sequence features of the BsMDH. A Secondary structure prediction of BsMDH. B Neighbor-joining phylogenetic tree of MDH amino acid sequences. Percentage bootstrap values above 50 are shown at the branch nodes. C Alignment of MDH protein sequences. The red color represents the conservation degree at each site. The accession numbers for the sequences in uniport database are as follows: EcMDH, Escherichia coli strain K12, P61889; PmMDH, pig mitochondria, P00346; RpMDH, Rickettsia prowazekii strain Madrid E, Q9ZDF3; SaMDH, Streptomyces aureofaciens, A0A1E7MZ09; ScMDH, Saccharomyces cerevisiae strain S288c, P22133; StMDH, Salmonella typhimurium strain LT2, P25077; TfMDH, Thermus flavus AT-62/Thermus thermophilus ATCC 33923, P10584 (Color figure online)
3.2 Effects of pH and Temperature on Enzyme Activity
Along with increasing temperature and pH, BsMDH activity increased from 20 to 38 °C and from pH 7.0 to 8.0, but then decreased. The optimal pH and temperature for BsMDH activity were 8.0 and 38 °C, respectively (Fig. 3A, B).
As shown in Fig. 3C, the residual activity of BsMDH maintained above 75% after treated at 30–40 °C for 20 min. When the temperature was above 40 °C, the residual enzyme activity of BsMDH decreased sharply with the gradually increased temperature. The residual activity was less than 20% of the initial enzyme activity after treatment at 55 °C for 20 min.
3.3 Effects of Metal Ions and Metabolic Reagents on BsMDH Activity
Treatments with 2 mM Rb+, Na+ and Li+ almost completely inhibited BsMDH enzyme activity. The activity of BsMDH decreased over 80% in the presence of 2 mM Co2+, Zn2+, Cu2+ and K+. Other metal ions at the concentration of 2 mM such as Mn2+, Mg2+, Ca2+, and Ni2+ also exhibited inhibitory activities on BsMDH activity, with the inhibitory rates ranging from 10 to 88%. These results indicated that BsMDH might be a metallic-ion-independent dehydrogenase, and its activity was inhibited by various metal ions (Table 1).
Regarding the effects of small molecular compounds, addition of 2 mM ATP, ADP and AMP inhibited BsMDH activity. Especially the addition of AMP inhibited almost 46% of BsMDH activity. Other small metabolic compounds, including α-KG, DTT and EDTA, slightly inhibited BsMDH activity, and the residual activity still maintained above 80% (Table 2).
3.4 Kinetic Parameters of BsMDH
As shown in Table 3, and Supplementary Fig. S2, Km and kcat of NADH were 54.97 µM and 86.10 s−1, respectively, and those of NADPH were 573.3 µM and 3.85 s−1, respectively. The catalytic efficiency (kcat/Km) values of BsMDH for NADH and NADPH were 1.57 µM−1 s− 1 and 0.007 µM−1 s−1, respectively. The Km value for OAA was 970.8 µM, and its catalytic efficiency (kcat/Km) was 0.32 µM−1 s−1 (Table 4, Supplementary Fig. S2).
3.5 Kinetic Analysis of Mutated Enzymes
Totally, six mutants on the key coenzyme binding site of BsMDH were constructed. The Circular Dichroism analysis showed that the curves of mutated proteins were basically consistent with that of the wild-type BsMDH (Supplementary Fig. S3), indicating that mutations at these sites did not significantly affect their secondary structure.
The Km values for NADH (KmNADH) and NADPH (KmNADPH) of the six mutants are listed in Table 5. Among them, the preference of BsMDH-T5 to NADPH was five times higher than to NADH, and coenzyme specificity to NADH of this mutant reduced from 224.3 to 0.2 (1122 folds). The coenzyme specificity of BsMDH-T7 to NADPH was 2039 folds higher than that of BsMDH, indicating the complete conversion of coenzyme specificity after mutation of certain amino acids. Notably, the other mutants, including BsMDH-T3, and BsMDH-T4, also showed great changes of kcat/Km, when the coenzyme changed from NADH to NADPH, with the degree of alteration of 329, and 575 folds, respectively.
4 Discussion
As previously reported, MDHs from Bacillus were predicted to be homotetramer based on the molecular mass analyses, including B. caldotenax [28], B. subtilis [29], and B. stearothermophilus [30]. Monomer state of MDH was also reported from another B. subtilis strain [29]. In addition, Wynne et al. [30] reported that the MDH from B. stearothermophilus could form dimers when the pH decreased below 3.5 but tetramer at pH higher than 3.5. Inconsistent with these results, in this study, both gel filtration and MALDI-TOF MS analyses indicated that BsMDH with his6-tag could form homodimer at pH 8.0. In addition, native-PAGE revealed two bands of BsMDH without his6-tag. MALDI-TOF MS analyses also revealed that BsMDH without his6-tag formed dimers. The GPC-MALS analysis revealed that the molecular weight of BsMDH was 123.1 kDa, which might be a mixture of dimer and tetramer. These results suggested that, in addition to tetramer, BsMDH might also form dimer, which is a novel finding and extends our knowledge on the oligomerization diversity of this enzyme. The inconsistent results between MALDI-TOF MS and GPC-MALS might be due to the different buffer systems, which required further investigations.
For the recombinantly expressed BsMDH, the kcat/Km value of OAA (0.32 µM−1 s−1) was higher than that of malate (0.095 µM−1 s−1), and the kcat/Km value of NADH (1.57 µM−1 s−1) was higher than that of NAD+ (0.20 µM−1 s−1). These results indicated that BsMDH might be more efficient to catalyze the reaction from OAA to malate than the reverse direction, which would benefit to the industrial synthesis of malate.
For enzymatic properties, the optimum pH for BsMDH activity was approximately 8.0, which was similar to MDH from Streptomyces aureofaciens (SaMDH) and MDH from Saccharomyces cerevisiae (ScMDH) [31, 32]. The thermal stability of MDHs was generally low, which could be improved by the increased number of hydrogen or disulfide bonds. Compared with MDHs of other species, thermophilic MDHs had more hydrogen bonds between the protein subunits of polar amino acid residues [33, 34]. MDHs from thermophilic microorganisms, i.e. ScMDH and NeMDH could retain most activity after treatment at 50 °C, but were completely inactivated after treatment at 60 °C [31, 35]. In the present study, BsMDH could retain approximately 75% of activity after treatment at 40 °C, but less than 20% of activity at 55 °C, indicating a moderate thermal stability of BsMDH. Comparison between different MDHs revealed that the affinity of thermophilic tetramer BaMDH (MDH from Bacillus sp.) to NADH and NADPH was higher than that of BsMDH (Km value), but the specificity of BsMDH to NADH was 2.87 times higher than those of BaMDH, ScMDH and TfMDH (MDH from Thermus flavus) [27, 30, 31] (Table 3).
Generally, the normal function of MDHs does not require metal ions. However, its activity may be affected by metal ions. The reductive activity of MDHs was inhibited by TCA cycle metabolites, such as excess oxaloacetate and divalent metal ions [5, 36]. In general, Ca2+, Mg2+ and most small molecular compounds did not greatly affect, but Zn2+ exhibited significant inhibitory effects on Phaseolus mungo MDH activity [37]. In the present study, similar results were observed. In contrast, K+, Co2+ and Ni2+ significantly promoted the activity of most MDH enzymes [36], but inhibited the activity of BsMDH in the present study. The underlying reasons were unknown and required further investigations.
α-KG, ATP, ADP and AMP are general metabolites in cells. DTT, and EDTA are commonly used components in buffer systems. Effects of these chemicals on BsMDH activity should be important for industrial application of BsMDH. As reported, rat MDH was inhibited by α-KG [38]. Activity of mitochondrial MDH from Arabidopsis thaliana was inhibited by AMP, ADP, and ATP, among which ATP showed the strongest inhibition [39]. Similar results were also observed in mitochondrial MDH from Solanum tuberosum [40]. Moreover, MDH activity from bacterium Nitrosomonas europaea was not strongly activated or inhibited by adenine nucleotides [26]. In the present study, α-KG, ADP and AMP showed remarkable inhibition to BsMDH. These results were consistent with the above publications, suggesting that application of BsMDH should avoid contamination of these molecules. In addition, DTT revealed moderate inhibition to BsMDH, probably due to the effects of DTT on disulfide bonds between BsMDH monomers, which are important to form dimers or tetramers.
As the kcat/Km values showed, in the presence of NADH, the catalytic efficiency of wide-type BsMDH was much higher than that using NADPH as the coenzyme, suggesting BsMDH relied on NADH as the coenzyme. This result was consistent with the most published bacterial MDHs [5]. As reported, only chloroplast MDHs were NADPH-dependent, while other MDHs, including LDH-like MDH, were NADH-dependent. Further tests of six mutated BsMDHs showed clear switch of coenzyme preference from NADH to NADPH, especially BsMDH-T5 and BsMDH-T7, displaying the degrees of alteration (the ratio of kcat/KmNADH of mutated enzyme to the kcat/KmNADPH of wide type) of 1121 and 2039 times, respectively. NADPH-dependent MDHs have been identified in nature. However, BsMDH-T7 generated in the present study revealed a lower Km value than wide-type NADPH-dependent MDHs from Thermus flavus (TfMDH), Corynebacterium glutamicum (CgMDH), Sorghum vulgare leaf chloroplasts (chMDH(SV)) and Zea mays chloroplasts (chMDH(ZM)). In addition, BsMDH-T7 showed higher kcat and kcat/Km values to NADPH than those of TfMDH and CgMDH, but lower than chMDH(SV) [27, 41,42,43,44] (Table 6). These results indicate that the created BsMDH-T7 mutant is a novel NADPH-dependent enzyme, which displayed better catalytic parameters than the reported NADPH-dependent MDHs, and might be potentially applied in industrial production.
As reported on dehydrogenase, cofactor binding sites are associated with arginine mutations [45, 46]. Similarly, in the present study, mutation on R39 (BsMDH-T1b) showed a degree of alteration of 4.47 in comparison to the wide type, and mutations on G36S37R39S40 (pBsMDH-T4) showed a higher degree of alteration than BsMDH-T3(G36S37S40), both indicating that R39 might be an important site for coenzyme preference. Arginine could interact with the 2′-phosphate group of adenine ribose of NADPH. In addition, replacement of Asp36 by Gly revealed promotive effects on preference switch from NADH to NADPH, probably due to the disappearance of electrostatic repulsion to NADPH and steric hindrance reported in several dehydrogenase [43, 47].
In summary, BsMDH may form both dimer and tetramer in vitro, depending on the buffer system. The optimum temperature and pH of BsMDH were 38 °C and pH 8.0, respectively. BsMDH shows a moderate thermal stability, and a higher efficacy in the catalysis direction of malate synthesis than the reverse. Mutations of certain amino acids at the coenzyme specific binding site change the coenzyme preference of BsMDH from NADH to NADPH. Among the mutants, BsMDH-T7 revealed a relatively lower Km value, but relatively higher kcat and kcat/Km than TfMDH and CgMDH. Overall, these results suggest that BsMDH and its mutant BsMDH-T7 are promising enzymes for malate production.
Data Availability
All data have been included in the manuscript and supplementary materials.
References
Rudrappa T, Czymmek KJ, Paré PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148(3):1547–1556
Gietl C (1992) Malate dehydrogenase isoenzymes: cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. Biochim Biophys Acta 1100(3):217–234
Meyer FM, Gerwig J, Hammer E, Herzberg C, Commichau FM, Völker U, Stülke J (2011) Physical interactions between tricarboxylic acid cycle enzymes in Bacillus subtilis: evidence for a metabolon. Metab Eng 13(1):18–27
Swinnen IA, Bernaerts K, Dens EJ, Geeraerd AH, Van Impe JF (2004) Predictive modelling of the microbial lag phase: a review. Int J Food Microbiol 94(2):137–159
Takahashi-Íñiguez T, Aburto-Rodríguez N, Vilchis-González AL, Flores ME (2016) Function, kinetic properties, crystallization, and regulation of microbial malate dehydrogenase. J Zhejiang Univ Sci B 17(4):247–261
Harrison JF, Nielsen DI, Page R (1996) Malate dehydrogenase phenotype, temperature and colony effects on flight metabolic rate in the honey-bee, Apis mellifera. Funct Ecol 10(1):81–88
Liu Z, Yuan F, Yang Y, Yin L, Liu Y, Wang Y, Zheng K, Cao J (2016) Partial protective immunity against toxoplasmosis in mice elicited by recombinant Toxoplasma gondii malate dehydrogenase. Vaccine 34(7):989–994
Thakker C, Martínez I, Li W, San K-Y, Bennett GN (2015) Metabolic engineering of carbon and redox flow in the production of small organic acids. J Ind Microbiol Biotechnol 42(3):403–422
Dong X, Chen X, Qian Y, Wang Y, Wang L, Qiao W, Liu L (2017) Metabolic engineering of Escherichia coli W3110 to produce l-malate. Biotechnol Bioeng 114(3):656–664
Geiser DM, Pitt JI, Taylor JW (1998) Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc Natl Acad Sci USA 95(1):388–393
Zhang X, Wang X, Shanmugam KT, Ingram LO (2011) l-Malate production by metabolically engineered Escherichia coli. Appl Environ Microbiol 77(2):427–434
Mu L, Wen J (2013) Engineered Bacillus subtilis 168 produces l-malate by heterologous biosynthesis pathway construction and lactate dehydrogenase deletion. World J Microbiol Biotechnol 29(1):33–41
Lin P, Yuan H, Du J, Liu K, Liu H, Wang T (2020) Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl Microbiol Biotechnol 104(6):2319–2331
Karpov DS, Domashin AI, Kotlov MI, Osipova PG, Kiseleva SV, Seregina TA, Goncharenko AV, Mironov AS, Karpov VL, Poddubko SV (2020) Biotechnological potential of the Bacillus subtilis 20 strain. Mol Biol 54(1):119–127
Kunst F et al (1997) The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390(6657):249–256
Lee NK, Kim WS, Paik HD (2019) Bacillus strains as human probiotics: characterization, safety, microbiome, and probiotic carrier. Food Sci Biotechnol 28(5):1297–1305
Olmos J, Acosta M, Mendoza G, Pitones V (2020) Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch Microbiol 202(3):427–435
Romero-Garcia S, Hernández-Bustos C, Merino E, Gosset G, Martinez A (2009) Homolactic fermentation from glucose and cellobiose using Bacillus subtilis. Microb Cell Fact 8(1):23
Kleijn RJ, Buescher JM, Le Chat L, Jules M, Aymerich S, Sauer U (2010) Metabolic fluxes during strong carbon catabolite repression by malate in Bacillus subtilis. J Biol Chem 285(3):1587–1596
Stülke J, Hillen W (2000) Regulation of carbon catabolism in Bacillus species. Annu Rev Microbiol 54:849–880
Lu Y, Mei L (2007) Co-expression of P450 BM3 and glucose dehydrogenase by recombinant Escherichia coli and its application in an NADPH-dependent indigo production system. J Ind Microbiol Biotechnol 34(3):247–253
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Ge YD, Jiang LL, Hou SL, Su FZ, Wang P, Zhang G (2020) Heteroexpression and biochemical characterization of thermostable citrate synthase from the cyanobacteria Anabaena sp. PCC7120. Protein Expr Purif 168:105565
Maloney AP, Callan SM, Murray PG, Tuohy MG (2004) Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii. Eur J Biochem 271(15):3115–3126
Deutch CE (2013) l-Malate dehydrogenase activity in the reductive arm of the incomplete citric acid cycle of Nitrosomonas europaea. Antonie Van Leeuwenhoek 104(5):645–655
Nishiyama M, Birktoft JJ, Beppu T (1993) Alteration of coenzyme specificity of malate dehydrogenase from Thermus flavus by site-directed mutagenesis. J Biol Chem 268(7):4656–4660
Smith K, Sundaram TK, Kernick M (1984) Malate dehydrogenases from actinomycetes: structural comparison of Thermoactinomyces enzyme with other actinomycete and Bacillus enzymes. J Bacteriol 157(2):684–687
Sundaram TK, Wright IP, Wilkinson AE (1980) Malate dehydrogenase from thermophilic and mesophilic bacteria. Molecular size, subunit structure, amino acid composition, immunochemical homology, and catalytic activity. Biochemistry 19(10):2017–2022
Wynne SA, Nicholls DJ, Scawen MD, Sundaram TK (1996) Tetrameric malate dehydrogenase from a thermophilic Bacillus: cloning, sequence and overexpression of the gene encoding the enzyme and isolation and characterization of the recombinant enzyme. Biochem J 317:235–245
Ge YD, Cao ZY, Wang ZD, Chen LL, Zhu YM, Zhu GP (2010) Identification and biochemical characterization of a thermostable malate dehydrogenase from the mesophile Streptomyces coelicolor A3(2). Biosci Biotechnol Biochem 74(11):2194–2201
Labrou NE, Clonis YD (1997) l-Malate dehydrogenase from Pseudomonas stutzeri: purification and characterization. Arch Biochem Biophys 337(1):103–114
Bjørk A, Dalhus B, Mantzilas D, Eijsink VG, Sirevåg R (2003) Stabilization of a tetrameric malate dehydrogenase by introduction of a disulfide bridge at the dimer–dimer interface. J Mol Biol 334(4):811–821
Jaindl M, Popp M (2006) Cyclitols protect glutamine synthetase and malate dehydrogenase against heat induced deactivation and thermal denaturation. Biochem Biophys Res Commun 345(2):761–765
Yoshida A (1965) Purification and chemical characterization of malate dehydrogenase of Bacillus subtilis. J Biol Chem 240:1113–1117
Takeya M, Ito S, Sukigara H, Osanai T (2018) Purification and characterisation of malate dehydrogenase from Synechocystis sp. PCC 6803: biochemical barrier of the oxidative tricarboxylic acid cycle. Front Plant Sci 9:947
Wang S, Xu Z, Ye X, Rao P (2005) Purification and characterization of a malate dehydrogenase from Phaseolus mungo. J Food Biochem 29(2):117–131
Fahien LA, Kmiotek EH, Macdonald MJ, Fibich B, Mandic M (1988) Regulation of malate dehydrogenase activity by glutamate, citrate, alpha-ketoglutarate, and multienzyme interaction. J Biol Chem 263(22):10687–10697
Yoshida K, Hisaboria T (2016) Adenine nucleotide-dependent and redox-independent control of mitochondrial malate dehydrogenase activity in Arabidopsis thaliana. Biochim Biophys Acta 1857(6):810–818
Rustin P, Valat M (1986) The control of malate dehydrogenase activity by adenine nucleotides in purified potato tuber (Solanum tuberosum L.) mitochondria. Arch Biochem Biophys 247(1):62–67
Genda T, Nakamatsu T, Ozak H (2003) Purification and characterization of malate dehydrogenase from Corynebacterium glutamicum. J Biosci Bioeng 95(6):562–566
Thompson H, Tersteegen A, Thauer RK, Hedderich R (1998) Two malate dehydrogenases in Methanobacterium thermoautotrophicum. Arch Microbiol 170(1):38–42
Lemaire M, Miginiac-Maslow M, Decottignies P (1996) The catalytic site of chloroplastic NADP-dependent malate dehydrogenase contains a His/Asp pair. Eur J Biochem 236(3):947–952
Kagawa T, Bruno PL (1988) NADP-malate dehydrogenase from leaves of Zea mays: purification and physical, chemical, and kinetic properties. Arch Biochem Biophys 260(2):674–695
Ashton AR, Trevanion SJ, Carr PD, Verger D, Ollis DL (2000) Structural basis for the light regulation of chloroplast NADP malate dehydrogenase. Physiol Plant 110(3):314–321
Birktoft JJ, Banaszak LJ (1983) The presence of a histidine-aspartic acid pair in the active site of 2-hydroxyacid dehydrogenases. X-ray refinement of cytoplasmic malate dehydrogenase. J Biol Chem 258(1):472–482
Chakraborty J, Nemeria NS, Farinas E, Jordan F (2018) Catalysis of transthiolacylation in the active centers of dihydrolipoamide acyltransacetylase components of 2-oxo acid dehydrogenase complexes. FEBS Open Bio 8(6):880–896
Acknowledgements
We would like to thank professor Guo-Ping Zhu (College of Life Sciences, Anhui Normal University, China), Anhui Provincial Key Laboratory of the Conservation and Exploitation of Biological Resources, Anhui Provincial Key Laboratory of Molecular Enzymology and Mechanism of Major Diseases, Key Laboratory of Biomedicine in Gene Diseases and Health of Anhui Higher Education Institutes for excellent technical support.
Funding
This work was supported by the National Natural Science Foundation of China [Grant Nos. 31300006, 32070463].
Author information
Authors and Affiliations
Contributions
YDG designed the experiments. FZS, LLJ and SLH collected samples, performed the comparative genomic analysis and analyzed the data. HHW and YTG drafted the manuscript and all authors revised it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ge, YD., Guo, YT., Jiang, LL. et al. Enzymatic Characterization and Coenzyme Specificity Conversion of a Novel Dimeric Malate Dehydrogenase from Bacillus subtilis. Protein J 42, 14–23 (2023). https://doi.org/10.1007/s10930-022-10087-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-022-10087-0