Abstract
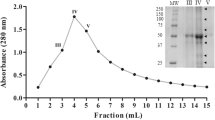
A new papain-like cysteine peptidase isolated from latex of Philibertia gilliesii Hook. et Arn., Apocynaceae (formerly Asclepiadaceae) has been purified and characterized. The enzyme, named philibertain g I, is the most basic component present in latex extracts and was purified by acetone fractionation followed by cation exchange chromatography (SP-Sepharose HR) using FPLC system. Homogeneity was confirmed by SDS-PAGE and mass spectroscopy (MS). Molecular mass of the enzyme was 23,530 Da (MALDI-TOF MS), its isoelectric point was >10.25, and maximum proteolytic activity (casein) was achieved at pH 7–8. The new protease was inhibited by E-64 a cysteine peptidases inhibitor. K m was 0.15 mM, using PFLNA as substrate. The N-terminal sequence of philibertain g I (LPASVDWRKEGAVLPIRHQGQCG) was compared with those of twenty plant proteases. Philibertain g I showed the higher degree of identity (73%) with caricain, one of the Carica papaya endopepetidases.
Similar content being viewed by others
Abbreviations
- AMPSO:
-
3-[(1,1-dimethyl-2-hydroxyethyl)amino]-2-hydroxy-propanesulfonic acid
- BLAST:
-
basic local alignment search tool
- CAPS:
-
3-(ciclohexylamino)-1-propanesulfonic acid
- DMSO:
-
dimethyl sulfoxide
- DTT:
-
dithiothreitol
- E-64:
-
trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane
- EDTA:
-
ethylendiaminetetraacetic acid
- IEF:
-
isoelectric focusing
- MALDI-TOF MS:
-
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MES:
-
2-(N-Morpholino)ethanesulfonic acid
- MOPS:
-
3-(N-morpholino) propanesulfonic acid
- PFLNA:
-
L-pyroglutamyl-L-phenylalanyl-L-leucine-p-nitroanilide
- PMSF:
-
phenylmethylsulfonyl fluoride
- SP-Sepharose:
-
sulphopropyl-Sepharose
- TAPS:
-
N-tris-(hydroxymethyl)-methyl-3-aminopropanesulfonic acid.
References
K. I. Abraham P. N. Joshi (1979) Biophys. Acta 568 111–119 Occurrence Handle1:CAS:528:DyaE1MXksFWltbw%3D
K. I. Abraham P. N. Joshi (1979) Biochim. Biophys. Acta 568 120–126 Occurrence Handle1:CAS:528:DyaE1MXksFWltb0%3D
H. Akasofu D. Yamauchi T. Minamikawa (1990) Nucleic Acids Res. 18 IssueID7 1892 Occurrence Handle1:CAS:528:DyaK3MXps1ShsQ%3D%3D
S. F. Altschul T. L. Madden A. A. Schaffer J. Zhang Z. Zhang W. Miller D. J. Lipman (1997) Nucleic Acids Res. 25 3389–3402 Occurrence Handle10.1093/nar/25.17.3389 Occurrence Handle1:CAS:528:DyaK2sXlvFyhu7w%3D
M. C. Arribére A. A. Cortadi M. A. Gattuso M. P. Bettiol N. S. Priolo N. O. Caffini (1998) Phytochem. Anal. 9 267–273
M. C. Arribére S. E. Vairo Cavalli N. S. Priolo N. O. Caffini (1999) Acta Horticult. 501 259–268
T. Asp S. Bowra S. Borg P. B. Holm (2004) Plant Sci. 167 825–837 Occurrence Handle10.1016/j.plantsci.2004.05.041 Occurrence Handle1:CAS:528:DC%2BD2cXmt1Wksr0%3D
B. E. Barragán M. T. Cruz L. M. del Castillo M. Castañeda-Agulló (1985) Rev. Latinoamer. Quim. 16 117–119
Boller, T. (1986). In: Dalling, M. J. (ed.), Plant Protelytic Enzymes. vol. I, CRC Press, Boca Raton, Florida, pp. 76–86.
M. M. Bradford (1976) Anal. Biochem. 72 248–254 Occurrence Handle10.1016/0003-2697(76)90527-3 Occurrence Handle1:CAS:528:DyaE28XksVehtrY%3D
W. J. Brockbank K. R. Lynn (1979) Biochim. Biophys. Acta 578 113–122
Correa, M. N. (1999). In: Flora Patagónica. Parte VI, Colección Cient., INTA, Buenos Aires.
T. Demura G. Tashiro G. Horiguchi N. Kishimoto M. Kubo N. Matsuoka A. Minami M. Nagata-Hiwatashi K. Nakamura Y. Okamura N. Sassa S. Suzuki J. Yazaki S. Kikuchi H. Fukuda (2002) Proc. Natl. Acad. Sci. U.S.A. 99 15794–15799 Occurrence Handle10.1073/pnas.232590499
M. E. Endress P. Bruyns (2000) Bot. Rev. 66 1–56 Occurrence Handle10.1007/BF02857781
I. Yu. Filippova E. N. Lysogorskaya E. S. Oksenoit G. N. Rudenskaya V. M. Stepanov (1984) Anal. Biochem. 143 293–297 Occurrence Handle10.1016/0003-2697(84)90665-1 Occurrence Handle1:CAS:528:DyaL2MXnsVGhsg%3D%3D
N. E. Good S. Izawa (1972) Meth. Enzymol. 24 53–68 Occurrence Handle1:CAS:528:DyaE3sXhtlCjug%3D%3D
K. Konno C. Hirayama M. Nakamura K. Tateishi Y. Tamura M. Hattori K. Kohno (2004) Plant. J. 37 370–378 Occurrence Handle10.1046/j.1365-313X.2003.01968.x Occurrence Handle1:CAS:528:DC%2BD2cXhvFejtr4%3D
S. Kundu M. Sundd M. V. Jagannadham (2000) J. Agric. Food Chem. 48 171–179 Occurrence Handle10.1021/jf990661j Occurrence Handle1:CAS:528:DC%2BD3cXmvV2nsQ%3D%3D
V. Kumar Dubey M. V. Jagannadham (2003) Phytochemistry 62 1057–1071 Occurrence Handle10.1016/S0031-9422(02)00676-3 Occurrence Handle1:CAS:528:DC%2BD3sXht1Crtro%3D
U. K. Laemmli (1970) Nature 227 680–685 Occurrence Handle10.1038/227680a0 Occurrence Handle1:CAS:528:DC%2BD3MXlsFags7s%3D
C. S. Liggieri M. C. Arribére S. A. Trejo F. Canals F. X. Aviles N. S. Priolo (2004) Protein J. 23 403–411 Occurrence Handle10.1023/B:JOPC.0000039554.18157.69 Occurrence Handle1:CAS:528:DC%2BD2cXntVKqsrk%3D
J. Liu L. A. Blaylock G. Endre J. Cho C. D. Town K. A. VandenBosch M. J. Harrison (2003) Plant Cell. 15 2106–2123 Occurrence Handle1:CAS:528:DC%2BD3sXnsV2gur0%3D
L. M. I. López C. Sequeiros C. L. Natalucci A. Brullo D. Maras B. Barra N. O. Caffini (2000) Protein Express. Purif. 18 133–140
K. R. Lynn W. J. Brockbank N. A. Clevette-Radford (1980) Biochim. Biophys. Acta 612 119–125 Occurrence Handle1:CAS:528:DyaL3cXhvFehtbc%3D
S. R. Morcelle S. A. Trejo F. Canals F. X. Aviles N. S. Priolo (2004) Protein J. 23 205–215 Occurrence Handle10.1023/B:JOPC.0000026416.90134.7b Occurrence Handle1:CAS:528:DC%2BD2cXjs1Kis7w%3D
Y. Naito M. Fujie S. Usami Y. Murooka T. Yamada (2000) Plant Physiol. 124 1087–1096 Occurrence Handle10.1104/pp.124.3.1087 Occurrence Handle1:CAS:528:DC%2BD3cXotlWru7g%3D
W. D. Obregón M. C. Arribére S. Morcelle del Valle C. Liggieri N. O. Caffini N. S. Priolo (2001) J. Protein Chem. 20 317–325
G. Pal N. K. Sinha (1980) Arch. Biochem. Biophys. 202 321–329 Occurrence Handle10.1016/0003-9861(80)90434-8 Occurrence Handle1:CAS:528:DyaL3cXkvFSktbs%3D
N. Priolo S. Morcelle del Valle M. C. Arribére L. M. I. López N. O. Caffini (2000) J. Protein Chem. 19 39–48 Occurrence Handle10.1023/A:1007042825783 Occurrence Handle1:CAS:528:DC%2BD3cXktl2mtLk%3D
Rawlings, N. M., and Barrett, A. J. (2004). In: Barrett, A. J., Rawlings, N. M., and Woessner, J. F. (eds.), Handbook of proteolytic enzymes. 2nd edn. Academic Press, London, Vol. II, pp. 1051–1071.
D. F. Revell N. J. Cummings K. C. Baker M. E. Collins M. A. Taylor I. G. Sumner R. W. Pickersgill I. F. Connerton P. W. Goodenough (1993) Gene 127 221–225 Occurrence Handle1:CAS:528:DyaK3sXks1Kmsbk%3D
C. Sakuta A. Oda M. Konishi S. Yamakawa H. Kamada S. Satoh (2001) Biosci. Biotechnol. Biochem. 65 2243–2248 Occurrence Handle10.1271/bbb.65.2243 Occurrence Handle1:CAS:528:DC%2BD3MXotFanu7w%3D
Salvesen, G., and Nagase, H. (2001). In: Beynon, R. J. and Bond, J. S. (eds.), Proteolytic Enzymes. Oxford University Press Inc., NY, pp. 112–122.
S. Sato Y. Nakamura T. Kaneko T. Katoh E. Asamizu H. Kotani S. Tabata (2000) DNA Res. 7 31–63 Occurrence Handle1:CAS:528:DC%2BD3cXhs1GnsbY%3D
M. Schmid D. Simpson F. Kalousek C. Gietl (1998) Planta 206 IssueID3 466–475 Occurrence Handle10.1007/s004250050423 Occurrence Handle1:CAS:528:DyaK1cXlvVeisro%3D
C. Sequeiros L. M. I. López N. O. Caffini C. L. Natalucci (2003) Fitoterapia 74 570–577 Occurrence Handle10.1016/S0367-326X(03)00146-1
M. Sundd S. Kundu G. P. Pal J. V. Medicherla (1998) Biosci. Biotechnol. Biochem. 62 1947–1955 Occurrence Handle10.1271/bbb.62.1947 Occurrence Handle1:STN:280:DyaK1M%2FltlWmsw%3D%3D
M. Tablero R. Arreguín B. Arreguín M. Soriano R. I. Sánchez A. Rodríguez-Romero A. Hernández-Arana (1991) Plant Sci. 74 7–15 Occurrence Handle10.1016/0168-9452(91)90250-C Occurrence Handle1:CAS:528:DyaK3MXktVektb8%3D
M. A. Taylor K. C. Baker G. S. Briggs I. F. Connerton N. J. Cummings K. A. Pratt D. F. Revell R. B. Freedman P. W. Goodenough (1995) Protein Eng. 8 IssueID1 59–62 Occurrence Handle1:CAS:528:DyaK2MXksVSjtrg%3D Occurrence Handle10.1093/protein/8.1.59
S. A. Trejo L. M. I. López C. V. Cimino N. O. Caffini C. L. Natalucci (2001) J. Protein Chem. 20 469–477 Occurrence Handle10.1023/A:1012502412612 Occurrence Handle1:CAS:528:DC%2BD3MXovVOqsrY%3D
S. E. Vairo Cavalli A. Cortadi M. C. Arribére P. Conforti N. O. Caffini N. S. Priolo (2001) Biol.Chem. Hoppe-Seyler 382 879–883
S. E. Vairo Cavalli M. C. Arribére A. Cortadi N. O. Caffini N. S. Priolo (2003) J. Protein Chem. 22 15–22 Occurrence Handle10.1023/A:1023059525861 Occurrence Handle1:CAS:528:DC%2BD3sXis1Cju7w%3D
L. Wan Q. Xia X. Qiu G. Selvaraj (2002) Plant J. 30 1–10 Occurrence Handle10.1046/j.1365-313X.2002.01262.x Occurrence Handle1:CAS:528:DC%2BD38XjvFGhurc%3D
J. L. Westergaar C. Hackbarth M. W. Treuhaft R. C. Roberts (1980) J. Immunol. Meth. 34 167–175
T. Winnick A. R. Davis D. M. Greenberg (1940) J. Gen. Physiol. 23 275–88 Occurrence Handle1:CAS:528:DyaH3cXjtFSqtg%3D%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sequeiros, C., Torres, M.J., Trejo, S.A. et al. Philibertain g I, the Most Basic Cysteine Endopeptidase Purified from the Latex of Philibertia gilliesii Hook. et Arn. (Apocynaceae). Protein J 24, 445–453 (2005). https://doi.org/10.1007/s10930-005-7640-0
Issue Date:
DOI: https://doi.org/10.1007/s10930-005-7640-0