Abstract
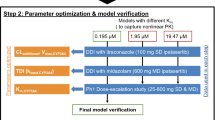
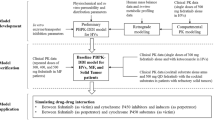
The new adjuvant chemotherapy of docetaxel, epirubicin, and cyclophosphamide has been recommended for treating breast cancer. It is necessary to investigate the potential drug-drug Interactions (DDIs) since they have a narrow therapeutic window in which slight differences in exposure might result in significant differences in treatment efficacy and tolerability. To guide clinical rational drug use, this study aimed to evaluate the DDI potentials of docetaxel, cyclophosphamide, and epirubicin in cancer patients using physiologically based pharmacokinetic (PBPK) models. The GastroPlus™ was used to develop the PBPK models, which were refined and validated with observed data. The established PBPK models accurately described the pharmacokinetics (PKs) of three drugs in cancer patients, and the predicted-to-observed ratios of all the PK parameters met the acceptance criterion. The PBPK model predicted no significant changes in plasma concentrations of these drugs during co-administration, which was consistent with the observed clinical phenomenon. Besides, the verified PBPK models were then used to predict the effect of other Cytochrome P450 3A4 (CYP3A4) inhibitors/inducers on these drug exposures. In the DDI simulation, strong CYP3A4 modulators changed the exposure of three drugs by 0.71–1.61 fold. Therefore, patients receiving these drugs in combination with strong CYP3A4 inhibitors should be monitored regularly to prevent adverse reactions. Furthermore, co-administration of docetaxel, cyclophosphamide, or epirubicin with strong CYP3A4 inducers should be avoided. In conclusion, the PBPK models can be used to further investigate the DDI potential of each drug and to develop dosage recommendations for concurrent usage by additional perpetrators or victims.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL et al (2022) Cancer statistics, 2022. CA: Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Nakatsukasa K et al (2017) Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer 24(1):63–68. https://doi.org/10.1007/s12282-016-0666-7
Marra A, Curigliano G (2021) Adjuvant and neoadjuvant treatment of triple-negative breast cancer with chemotherapy. Cancer J 27(1):41–49. https://doi.org/10.1097/ppo.0000000000000498
Baker J et al (2009) Docetaxel-related side effects and their management. Eur J Oncol Nurs 13(1):49–59. https://doi.org/10.1016/j.ejon.2008.10.003
Nicolella D et al (1996) Weekly low dose epirubicin in elderly cancer patients. Tumori 82(4):369–371. https://doi.org/10.1177/030089169608200414
Gori S et al (2006) Safety of epirubicin adjuvant chemotherapy in a breast cancer patient with chronic renal failure undergoing hemodialytic treatment. Tumori 92(4):364–365. https://doi.org/10.1177/030089160609200421
Morgan GJ, Davies FE (2013) Role of thalidomide in the treatment of patients with multiple myeloma. Crit Rev Oncol Hematol 88(Suppl 1):S14-22. https://doi.org/10.1016/j.critrevonc.2013.05.012
Tamura T et al (2016) A phase i trial of 100 mg/m2 docetaxel in patients with advanced or recurrent breast cancer. Acta Med Okayama 70(5):425–427. https://doi.org/10.18926/amo/54607
Muth M et al (2021) Role of TDM-based dose adjustments for taxane anticancer drugs. Br J Clin Pharmacol 87(2):306–316. https://doi.org/10.1111/bcp.14678
Baker SD, Sparreboom A, Verweij J (2006) Clinical pharmacokinetics of docetaxel: recent developments. Clin Pharmacokinet 45(3):235–252. https://doi.org/10.2165/00003088-200645030-00002
Clarke SJ, Rivory LP (1999) Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 36(2):99–114. https://doi.org/10.2165/00003088-199936020-00002
Shirakawa K et al (1999) Interaction of docetaxel (“Taxotere”) with human P-glycoprotein. Jpn J Cancer Res 90(12):1380–1386. https://doi.org/10.1111/j.1349-7006.1999.tb00723.x
Dyshlovoy SA et al (2022) New diterpenes from the marine sponge Spongionella sp. overcome drug resistance in prostate cancer by inhibition of P-glycoprotein. Sci Rep 12(1):13570. https://doi.org/10.1038/s41598-022-17447-x
Vredenburg G et al (2015) Activation of the anticancer drugs cyclophosphamide and ifosfamide by cytochrome P450 BM3 mutants. Toxicol Lett 232(1):182–192. https://doi.org/10.1016/j.toxlet.2014.11.005
de Jonge ME et al (2005) Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 44(11):1135–1164. https://doi.org/10.2165/00003088-200544110-00003
Yule SM et al (2001) Cyclophosphamide metabolism in children following a 1-h and a 24-h infusion. Cancer Chemother Pharmacol 47(3):222–228. https://doi.org/10.1007/s002800000220
Martin H et al (2003) Induction of cytochrome P450 2B6 and 3A4 expression by phenobarbital and cyclophosphamide in cultured human liver slices. Pharm Res 20(4):557–568. https://doi.org/10.1023/a:1023234429596
Cersosimo RJ, Hong WK (1986) Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue. J Clin Oncol 4(3):425–439. https://doi.org/10.1200/jco.1986.4.3.425
Tariq M et al (2016) Improved oral efficacy of epirubicin through polymeric nanoparticles: pharmacodynamic and toxicological investigations. Drug Deliv 23(8):2990–2997. https://doi.org/10.3109/10717544.2015.1136713
Liu K et al (2021) Melatonin increases the chemosensitivity of diffuse large B-cell lymphoma cells to epirubicin by inhibiting P-glycoprotein expression via the NF-κB pathway. Transl Oncol 14(1):100876. https://doi.org/10.1016/j.tranon.2020.100876
Lindley C et al (2002) The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metab Dispos 30(7):814–822. https://doi.org/10.1124/dmd.30.7.814
Eksborg S (1989) Pharmacokinetics of anthracyclines. Acta Oncol 28(6):873–876. https://doi.org/10.3109/02841868909092323
Ismail M et al (2020) Prevalence and significance of potential drug-drug interactions among cancer patients receiving chemotherapy. BMC Cancer 20(1):335. https://doi.org/10.1186/s12885-020-06855-9
Rodrigues J et al (2023) Mitigating the risk of drug interactions in cancer patients taking oral anticancer agents: the role of a multidisciplinary team-based medication reconciliation. Cureus 15(2):e35324. https://doi.org/10.7759/cureus.35324
Mueller-Schoell A et al (2021) Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol 77(4):441–464. https://doi.org/10.1007/s00228-020-03014-8
Fahmy A et al (2021) Evaluating the utility of therapeutic drug monitoring in the clinical use of small molecule kinase inhibitors: a review of the literature. Expert Opin Drug Metab Toxicol 17(7):803–821. https://doi.org/10.1080/17425255.2021.1943357
Lin W et al (2022) Applications, challenges, and outlook for PBPK modeling and simulation: a regulatory. Ind Acad Perspect Pharm Res 39(8):1701–1731. https://doi.org/10.1007/s11095-022-03274-2
Kuepfer L et al (2016) Applied concepts in PBPK modeling: how to build a PBPK/PD model. CPT Pharmacometrics Syst Pharmacol 5(10):516–531. https://doi.org/10.1002/psp4.12134
Rowland Yeo K et al (2011) Prediction of time-dependent CYP3A4 drug-drug interactions by physiologically based pharmacokinetic modelling: impact of inactivation parameters and enzyme turnover. Eur J Pharm Sci 43(3):160–173. https://doi.org/10.1016/j.ejps.2011.04.008
Sudsakorn S et al (2020) 2020 FDA drug-drug interaction guidance: a comparison analysis and action plan by pharmaceutical industrial scientists. Curr Drug Metab 21(6):403–426. https://doi.org/10.2174/1389200221666200620210522
Mendes MS et al (2020) A physiologically based pharmacokinetic—pharmacodynamic modelling approach to predict incidence of neutropenia as a result of drug-drug interactions of paclitaxel in cancer patients. Eur J Pharm Sci 150:105355. https://doi.org/10.1016/j.ejps.2020.105355
Cheeti S et al (2013) A physiologically based pharmacokinetic (PBPK) approach to evaluate pharmacokinetics in patients with cancer. Biopharm Drug Dispos 34(3):141–154. https://doi.org/10.1002/bdd.1830
Baker SD et al (2004) Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res 10(24):8341–8350. https://doi.org/10.1158/1078-0432.Ccr-04-1371
Peng B et al (2004) Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 22(5):935–942. https://doi.org/10.1200/jco.2004.03.050
Joseph VR (2022) Optimal ratio for data splitting. Statist Analy Data Mining 15(4):531–538. https://doi.org/10.1002/sam.11583
Singh A et al (2021) Novel vitamin E TPGS based docetaxel nanovesicle formulation for its safe and effective parenteral delivery: toxicological, pharmacokinetic and pharmacodynamic evaluation. J Liposome Res 31(4):365–380. https://doi.org/10.1080/08982104.2020.1835955
Bruno R, Sanderink GJ (1993) Pharmacokinetics and metabolism of taxotere (docetaxel). Cancer Surv 17:305–313
Rodgers T, Leahy D, Rowland M (2005) Tissue distribution of basic drugs: accounting for enantiomeric, compound and regional differences amongst beta-blocking drugs in rat. J Pharm Sci 94(6):1237–1248. https://doi.org/10.1002/jps.20323
Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95(6):1238–1257. https://doi.org/10.1002/jps.20502
Han SY et al (2019) LC478, a novel di-substituted adamantyl derivative, enhances the oral bioavailability of docetaxel in rats. Pharmaceutics 11(3):135. https://doi.org/10.3390/pharmaceutics11030135
Ansaar R, Meech R, Rowland A (2023) A physiologically based pharmacokinetic model to predict determinants of variability in epirubicin exposure and tissue distribution. Pharmaceutics 15(4):1222. https://doi.org/10.3390/pharmaceutics15041222
Sparreboom A et al (1998) Preclinical pharmacokinetics of paclitaxel and docetaxel. Anticancer Drugs 9(1):1–17. https://doi.org/10.1097/00001813-199801000-00001
Cai T et al (2020) The influence of different triazole antifungal agents on the pharmacokinetics of cyclophosphamide. Ann Pharmacother 54(7):676–683. https://doi.org/10.1177/1060028019896894
Robert J (1994) Clinical pharmacokinetics of epirubicin. Clin Pharmacokinet 26(6):428–438. https://doi.org/10.2165/00003088-199426060-00002
Cohen JL, Jao JY, Jusko WJ (1971) Pharmacokinetics of cyclophosphamide in man. Br J Pharmacol 43(3):677–680. https://doi.org/10.1111/j.1476-5381.1971.tb07199.x
Shou M et al (1998) Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics 8(5):391–401. https://doi.org/10.1097/00008571-199810000-00004
Huang Z, Roy P, Waxman DJ (2000) Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol 59(8):961–972. https://doi.org/10.1016/s0006-2952(99)00410-4
Iwakiri T et al (2008) Inhibition of carrier-mediated uptake of epirubicin reduces cytotoxicity in primary culture of rat hepatocytes. J Appl Toxicol 28(3):329–336. https://doi.org/10.1002/jat.1283
Baumhäkel M et al (2001) Screening for inhibitory effects of antineoplastic agents on CYP3A4 in human liver microsomes. Int J Clin Pharmacol Ther 39(12):517–528. https://doi.org/10.5414/cpp39517
Hedmer M et al (2008) Validation of urinary excretion of cyclophosphamide as a biomarker of exposure by studying its renal clearance at high and low plasma concentrations in cancer patients. Int Arch Occup Environ Health 81(3):285–293. https://doi.org/10.1007/s00420-007-0211-2
Shin DH et al (2014) Hepatic uptake of epirubicin by isolated rat hepatocytes and its biliary excretion after intravenous infusion in rats. Arch Pharm Res 37(12):1599–1606. https://doi.org/10.1007/s12272-014-0475-5
Innocenti F et al (2001) Epirubicin glucuronidation is catalyzed by human UDP-glucuronosyltransferase 2B7. Drug Metab Dispos 29(5):686–692
Zhao P (2017) Report from the EMA workshop on qualification and reporting of physiologically based pharmacokinetic (PBPK) modeling and simulation. CPT Pharmacometrics Syst Pharmacol 6(2):71–72. https://doi.org/10.1002/psp4.12166
Abduljalil K et al (2014) Deciding on success criteria for predictability of pharmacokinetic parameters from in vitro studies: an analysis based on in vivo observations. Drug Metab Dispos 42(9):1478–1484. https://doi.org/10.1124/dmd.114.058099
Finch A, Pillans P (2014) P-glycoprotein and its role in drug-drug interactions. Aust Prescriber 37:137–139. https://doi.org/10.18773/austprescr.2014.050
Liu H et al (2021) Application of physiologically based pharmacokinetic modeling to evaluate the drug-drug and drug-disease interactions of apatinib. Front Pharmacol 12:780937. https://doi.org/10.3389/fphar.2021.780937
Davis MW, Wason S, Digiacinto JL (2013) Colchicine-antimicrobial drug interactions: what pharmacists need to know in treating gout. Consult Pharm 28(3):176–183. https://doi.org/10.4140/TCP.n.2013.176
Oo C, Chen YC (2009) The need for multiple doses of 400 mg ketoconazole as a precipitant inhibitor of a CYP3A substrate in an in vivo drug-drug interaction study. J Clin Pharmacol 49(3):368–369. https://doi.org/10.1177/0091270008325931
Ke AB et al (2014) Itraconazole and clarithromycin as ketoconazole alternatives for clinical CYP3A inhibition studies. Clin Pharmacol Ther 95(5):473–476. https://doi.org/10.1038/clpt.2014.41
Khadka P et al (2018) Considerations in preparing for clinical studies of inhaled rifampicin to enhance tuberculosis treatment. Int J Pharm 548(1):244–254. https://doi.org/10.1016/j.ijpharm.2018.07.011
Kenmotsu H, Tanigawara Y (2015) Pharmacokinetics, dynamics and toxicity of docetaxel: why the Japanese dose differs from the Western dose. Cancer Sci 106(5):497–504. https://doi.org/10.1111/cas.12647
Vasey PA et al (2002) Phase I study of docetaxel in combination with cyclophosphamide as first-line chemotherapy for metastatic breast cancer. Br J Cancer 87(10):1072–1078. https://doi.org/10.1038/sj.bjc.6600626
Rischin D et al (2002) Phase I and pharmacokinetic study of docetaxel in combination with epirubicin and cyclophosphamide in advanced cancer: dose escalation possible with granulocyte colony-stimulating factor, but not with prophylactic antibiotics. Ann Oncol 13(11):1810–1818. https://doi.org/10.1093/annonc/mdf305
Engels FK et al (2004) Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther 75(5):448–454. https://doi.org/10.1016/j.clpt.2004.01.001
Grimstein M et al (2019) Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration’s Office of clinical pharmacology. J Pharm Sci 108(1):21–25. https://doi.org/10.1016/j.xphs.2018.10.033
Wagner C et al (2015) Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin Pharmacokinet 54(1):117–127. https://doi.org/10.1007/s40262-014-0188-4
Wagner C et al (2016) Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin Pharmacokinet 55(4):475–483. https://doi.org/10.1007/s40262-015-0330-y
Alevizakos M et al (2016) Colonisation with extended-spectrum β-lactamase-producing enterobacteriaceae and risk for infection among patients with solid or haematological malignancy: a systematic review and meta-analysis. Int J Antimicrob Agents 48(6):647–654. https://doi.org/10.1016/j.ijantimicag.2016.08.021
Gudiol C, Aguado JM, Carratalà J (2016) Bloodstream infections in patients with solid tumors. Virulence 7(3):298–308. https://doi.org/10.1080/21505594.2016.1141161
Baneyx G et al (2014) Physiologically based pharmacokinetic modeling of CYP3A4 induction by rifampicin in human: influence of time between substrate and inducer administration. Eur J Pharm Sci 56:1–15. https://doi.org/10.1016/j.ejps.2014.02.002
Ma Y et al (2021) Therapeutic drug monitoring of docetaxel by pharmacokinetics and pharmacogenetics: a randomized clinical trial of AUC-guided dosing in nonsmall cell lung cancer. Clin Transl Med 11(4):e354. https://doi.org/10.1002/ctm2.354
(2007) American Society of Clinical Oncology 2007 clinical practice guideline recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Oncol Pract 3(6), 326–329. https://doi.org/10.1200/jop.0768502
Thai HT et al (2015) Optimizing pharmacokinetic bridging studies in paediatric oncology using physiologically-based pharmacokinetic modelling: application to docetaxel. Br J Clin Pharmacol 80(3):534–547. https://doi.org/10.1111/bcp.12702
Acknowledgements
We are grateful to all people for the contributions to the manuscript.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
T.T. Li: Conceptualization, Methodology, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing. S.F. Zhou: Conceptualization, Methodology, Investigation, Writing—Review & Editing, Supervision. L. Wang: Conceptualization, Methodology, Investigation, Supervision. T.P. Zhao: Conceptualization, Investigation, Methodology. J. Wang: Conceptualization, Methodology, Investigation. F. Shao: Conceptualization, Writing—Review & Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, T., Zhou, S., Wang, L. et al. Docetaxel, cyclophosphamide, and epirubicin: application of PBPK modeling to gain new insights for drug-drug interactions. J Pharmacokinet Pharmacodyn (2024). https://doi.org/10.1007/s10928-024-09912-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10928-024-09912-z