Abstract
Purpose
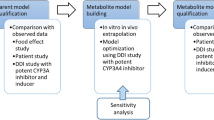
This study aimed to investigate the drug–drug interactions of ponatinib with strong, moderate, or weak CYP3A4 inhibitors/inducers by developing physiologically based pharmacokinetic (PBPK) models.
Methods
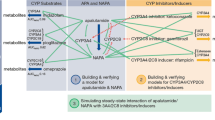
Simcyp® Ver 20.1 (Certara Inc., Sheffield, UK) was used to construct a PBPK model for ponatinib and to predict its interaction with strong, moderate, or weak CYP3A4 inhibitors/inducers. The constructed model was validated by comparing predicted values with actual observed values. Inhibitors or inducers that increased or decreased the area under the plasma concentration curve of ponatinib by more than two-fold when used in combination were considered significant.
Results
The PBPK model of ponatinib accurately represented its oral pharmacokinetics. It also reasonably predicted its pharmacokinetics when combined with ketoconazole and rifampicin. No weak to strong CYP3A4 inhibitor combinations significantly increased the AUC of ponatinib. However, the strong CYP3A4 inducers rifampicin (oral, 600 mg QD) and phenytoin (oral, 100 mg TID) decreased AUC by 60–70% and 50%, respectively.
Conclusions
The PBPK model predicted a significant drug interaction when ponatinib was combined with a strong CYP3A4 inducer. Conversely, the combination with weak-to-strong CYP3A4 inhibitors did not suggest a drug interaction with ponatinib.



Similar content being viewed by others
Code availability
None.
References
O’Hare T, Shakespeare WC, Zhu X et al (2009) AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 16(5):401–412
Deininger MW, Hodgson JG, Shah NP et al (2016) Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood 127(6):703–712
Baccarani M, Deininger MW, Rosti G et al (2013) European leukemia net recommendations for the management of chronic myeloid leukemia: 2013. Blood 122(6):872–884
Clinical Pharmacology and Biopharmaceutics Review(s): Iclusig®(ponatinib tablets). Pharmaceuticals and Medical Devices Agency in Japan. https://www.pmda.go.jp/drugs/2016/P20161021002/index.html
Narasimhan NI, Dorer DJ, Davis J et al (2014) Evaluation of pharmacokinetics and safety of ponatinib in subjects with chronic hepatic impairment and matched healthy subjects. Cancer Chemother Pharmacol 74:341–348
Narasimhan NI, Dorer DJ, Niland K et al (2013) Effects of ketoconazole on the pharmacokinetics of ponatinib in healthy subjects. J Clin Pharmacol 53(9):974–981
Narasimhan NI, Dorer DJ, Davis J et al (2015) Evaluation of the effect of multiple doses of rifampin on the pharmacokinetics and safety of ponatinib in healthy subjects. Clin Pharmacol Drug Dev 4(5):354–360
Tojo A, Kyo T, Yamamoto K, Nakamae H et al (2017) Ponatinib in Japanese patients with Philadelphia chromosome-positive leukemia, a phase 1/2 study. Int J Hematol 106:385–397
Dorer DJ, Knickerbocker RK, Baccarani M et al (2016) Impact of dose intensity of ponatinib on selected adverse events: Multivariate analyses from a pooled population of clinical trial patients. Leuk Res 48:84–91
Larson RA (2018) Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma 59:3–13
Assessment report: Iclusig®(ponatinib tablets). European Medicines Agency. https://www.ema.europa.eu/en/documents/assessment-report/iclusig-epar-public-assessment-report_en.pdf.
Ye YE, Woodward CN, Narasimhan NI (2017) Absorption, metabolism, and excretion of [14 C] ponatinib after a single oral dose in humans. Cancer Chemother Pharmacol 79(3):507–518
Ono C, Hsyu PH, Abbas R et al (2017) Application of physiologically based pharmacokinetic modeling for the understanding of bosutinib pharmacokinetics: prediction of drug–drug and drug-disease interactions. Drug Metab Dispos 45(4):390–398
Rowland Yeo K, Jamei M, Yang J, A. et al (2010) Physiologically based mechanistic modelling to predict complex drug–drug interactions involving simultaneous competitive and time dependent enzyme inhibition by parent compound and its metabolite in both liver and gut — the effect of diltiazem on the time-course of exposure to triazolam. Eur J Pharm Sci. 39:298–309
Friedman EJ, Fraser IP, Wang YH et al (2011) Effect of different durations and formulations of diltiazem on the single-dose pharmacokinetics of midazolam: how long do we go? J Clin Pharmacol 51:1561–1570
Zhang X, Quinney SK, Gorski JC et al (2009) Semiphysiologically based pharmacokinetic models for the inhibition of midazolam clearance by diltiazem and its major metabolite. Drug Metab Dispos 37:1587–1597
Li X, Junge L, Taubert M, von Georg A et al (2020) A novel study design using continuous intravenous and intraduodenal infusions of midazolam and voriconazole for mechanistic quantitative assessment of hepatic and intestinal CYP3A inhibition. J Clin Pharmacol 60:1237–1253
Lee S, Kim BH, Nam WS et al (2012) Effect of CYP2C19 polymorphism on the pharmacokinetics of voriconazole after single and multiple doses in healthy volunteers. J Clin Pharmacol 52:195–203
Drug Development and Drug Interactions: U.S. Food and Drug Administration. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers
Hanley MJ, Diderichsen PM, Narasimhan N et al (2021) Population pharmacokinetics of ponatinib in healthy adult volunteers and patients with hematologic malignancies and model-informed dose selection for pediatric development. J Clin Pharmacol. 62(4):555–567
Acknowledgements
This work was supported in part by the Foundation of Cancer Research in Japan.
Funding
The Foundation for the Promotion of Cancer Research in Japan supported this study (Grant number: FPCR-2022-B).
Author information
Authors and Affiliations
Contributions
TOM: study planning, conducting research, data analysis. KH: study planning.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Each patient provided written informed consent to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morita, T.O., Hanada, K. Physiologically based pharmacokinetic modeling of ponatinib to describe drug–drug interactions in patients with cancer. Cancer Chemother Pharmacol 90, 315–323 (2022). https://doi.org/10.1007/s00280-022-04466-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04466-8