Abstract
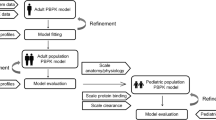
Limited pharmacokinetic (PK) and pharmacodynamic (PD) data are available to use in methadone dosing recommendations in pediatric patients for either opioid abstinence or analgesia. Considering the extreme inter-individual variability of absorption and metabolism of methadone, population-based PK would be useful to provide insight into the relationship between dose, blood concentrations, and clinical effects of methadone. To address this need, an age-dependent physiologically based pharmacokinetic (PBPK) model has been constructed to systematically study methadone metabolism and PK. The model will facilitate the design of cost-effective studies that will evaluate methadone PK and PD relationships, and may be useful to guide methadone dosing in children. The PBPK model, which includes whole-body multi-organ distribution, plasma protein binding, metabolism, and clearance, is parameterized based on a database of pediatric PK parameters and data collected from clinical experiments. The model is further tailored and verified based on PK data from individual adults, then scaled appropriately to apply to children aged 0–24 months. Based on measured variability in CYP3A enzyme expression levels and plasma orosomucoid (ORM2) concentrations, a Monte–Carlo-based simulation of methadone kinetics in a pediatric population was performed. The simulation predicts extreme variability in plasma concentrations and clearance kinetics for methadone in the pediatric population, based on standard dosing protocols. In addition, it is shown that when doses are designed for individuals based on prior protein expression information, inter-individual variability in methadone kinetics may be greatly reduced.
Similar content being viewed by others
References
Lugo R.A., MacLaren R., Cash J., Pribble C.G., Vernon D.D., (2001). Enteral to expedite fentanyl discontinuation and prevent opioid abstinence syndrome in the PICU. Pharmacotherapy 21:1566–1573
Tobias J.D., (2000). Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit. Care. Med. 28:2122–2132
Tobias J.D., Schleien C.L., Haun S.E., (1990). Methadone as treatment for iatrogenic narcotic dependency in pediatric intensive care unit patients. Crit. Care. Med. 18:1292–1293
Garrido M.J., Jiminez R., Gomez E., Calvo R., (1996). Influence of plasma-protein on analgesic effect of methadone in rats with spontaneous withdrawal. Pharm J., Pharmacol. 48:281–284
Abramson F.P., (1982). Methadone plasma protein binding: alterations in cancer and from alpha 1-acid glycoprotein. Clin. Pharmacol. Ther. 32:652–658
de Vos J.W., Geerlings P.J., van den Brink W., Ufkes J.G., van Wilgenburg H. (1995). Pharmacokinetics of methadone and its primary metabolite in 20 opiate addicts. Eur. J. Clin. Pharmacol. 48:361–366
Kharasch E.D., Hoffer C., Whittington D., Sheffels P., (2004). Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition, and miotic effects of methadone. Clin. Pharmacol. Ther. 76:250–269
Wang J.S., DeVane C.L., (2003). Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab. Dispos. 31:742–747
Wong S.H., Wagner M.A., Jentzen J.M., Schur C., Bjerke J., Gock S.B., Chang C. C., (2003). Pharmacogenomics as an aspect of molecular autopsy for forensic pathology/toxicology: does genotyping CYP 2D6 serve as an adjunct for certifying methadone toxicity?. J. Forensic. Sci. 48:1406–1415
Kristensen K., Blemmer T., Angelo H.R., Christrup L.L., Drenck N.E., Rasmussen S.N., Sjogren P., (1996). Stereoselective pharmacokinetics of methadone in chronic pain patients. Ther. Drug. Monit. 18:221–227
Boulton D.W., Arnaud P., DeVane C.L., (2001). Pharmacokinetics and pharmacodynamics of methadone enantiomers after a single oral dose of racemate. Clin. Pharmacol. Ther. 70:48–57
Wolff K., Hay A.W., Raistrick D., Calvert R., (1993). Steady-state pharmacokinetics of methadone in opioid addicts. Eur. J. Clin. Pharmacol. 44:189–194
Anggard E., Gunne L.M., Homstrand J., McMahon R.E., Sandberg C.G., Sullivan H.R., (1975). Disposition of methadone in methadone maintenance. Clin. Pharmacol. Ther. 17:258–266
Inturrisi C.E., Verebely K., (1972). Disposition of methadone in man after a single oral dose. Clin. Pharmacol. Ther. 13:923–930
Robinson A.E., Williams F.M., (1971). The distribution of methadone in man. J. Pharm. Pharmacol. 23:353–358
Iribarne C., Dreano Y., Bardou L.G., Menez J.F., Berthou F., (1997). Interaction of methadone with substrates of human hepatic cytochrome P450 3A4. Toxicology 117:13–23
Lotsch J., Skarke C., Tegeder I., Geisslinger G., (2002). Drug interactions with patient-controlled analgesia. Clin. Pharmacokinet. 41:31–57
Ferrari A., Coccia C.P., Bertolini A., Sternieri E., (2004).Methadone–metabolism, and interactions. Pharmacol. Res. 50:551–559
Bruera E., Sweeney C., (2002). Methadone use in cancer patients with pain: a review. J. Palliat. Med. 5:127–138
Rachel H., Robert A., Jason W., Wayne H., and Michael F., Proceedings of the expert workshop on induction and stabilisation of patients onto methadone in Findings of an expert workshop, Adelaide, South Australia. ISBN 0642415080 (2000).
Garrido M.J., Troconiz I.F.,(1999). Methadone: a review of its pharmacokinetic/ properties. J. Pharmacol. Toxicol. Methods 42:61–66
Taj R., Keenan E., O’Connor J.J., (1995). A review of patients on methadone Ir. Med. J. 88:218–219
Gabrielsson J.L., Johansson P., Bondesson U., Paalzow L.K., (1985). Analysis of methadone disposition in the pregnant rat by means of a physiological flow model. J. Pharmacokinet. Biopharm. 13:355–372
Eap C.B., Buclin T., Baumann P., (2002). Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin. Pharmacokinet. 41:1153–1193
Walsh C.T., Levine R.R., Squires C., (1975). The gastrointestinal absorption of in the rat. Drug Metab. Dispos. 3:525–529
Ito K., Iwatsubo T., Kanamitsu S., Ueda K., Suzuki H., Sugiyama Y., (1998). Prediction of pharmacokinetic alterations caused by drug-drug interactions: metabolic interaction in the liver. Pharmacol. Rev. 50:387–412
Foster D.J., Somogyi A.A., Dyer K.R., White J.M., Bochner F., (2000). Steady-state pharmacokinetics of (R)- and (S)-methadone in methadone maintenance patients. Br. J. Clin. Pharmacol. 50:427–440
Foster D.J., Somogyi A.A., Bochner F., (1999). Methadone N-demethylation in human liver microsomes: lack of stereoselectivity and involvement of CYP3A4. Br. J. Clin. Pharmacol. 47:403–412
Peck D.G., Beckett W., (1976). Methadone maintenance: a review and critique. Br. J. Addict Alcohol Other Drugs 71:369–376
Stevens J.C., Hines R.N., Gu C., Koukouritaki S.B., Manro J.R., Tandler P.J., Zaya M.J., (2003). Developmental expression of the major human hepatic CYP3A enzymes. J. Pharmacol. Exp. Ther. 307:573–582
Kuehl P., Zhang J., Lin Y., Lamba J., Assem M., Schuetz J., Watkins P.B., Daly A., Wrighton S.A., Hall S.D., Maurel P., Relling M., Brimer C., Yasuda K.,Strom R S., Thummel K., Boguski M.S., Schuetz E., (2001). Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 27:383–391
Nilsson M.I., Meresaar U., Anggard E., (1982). Clinical pharmacokinetics of methadone. Acta. Anaesthesiol. Scand. Suppl. 74:66–69
Charnick S.B., Kawai R., Nedelman J.R., Lemaire M., Niederberger W., Sato H., (1995). Perspectives in pharmacokinetics. Physiologically based pharmacokinetic modeling as a tool for drug development. J. Pharmacokinet. Biopharm. 23:217–229
Rowland M., Balant L., Peck C., Physiologically Based pharmacokinetics in drug development and regulatory science: a workshop report (Georgetown University, Washington, DC, May 29–30, 2002). AAPS PharmSci. 6:E6 (2004).
McNamara P.J., Alcorn J., (2002).Protein binding predictions in infants. AAPS 4:E4
McCarver D.G., Hines R.N., (2002). The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J. Pharmacol. Exp. Ther. 300:361–366
Hines R.N., McCarver D.G., (2002). The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J. Pharmacol. Exp. Ther. 300:355–360
Bjorkman S., (2005). Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br. J. Clin. Pharmacol. 59:691–704
Ginsberg G., Hattis D., Russ A., Sonawane B., (2004). Physiologically based (PBPK) modeling of caffeine and theophylline in neonates and adults: implications for assessing children’s risks from environmental agents. J. Toxicol. Environ. Health A 67:297–329
Price P.S., Conolly R.B., Chaisson C.F., Gross E.A., Young J.S., Mathis E.T., Tedder D.R., (2003). Modeling interindividual variation in physiological factors used in PBPK models of humans Crit. Rev. Toxicol. 33:469–503
ICRP 2002 Basic anatomical and physiological data for use in radiological protection: reference values ICRP. Publication 89 (ISSN: 0146 6453).
Haddad S., Restieri C., Krishnan K., (2001). Characterization of age-related changes in body weight and organ weights from birth to adolescence in humans. J. Toxicol. Health A 64:453–464
Boffito M., Sciole K., Raiteri R., Bonora S., Hoggard P.G., Back D.J., Di Perri G. (2002). Alpha 1-acid glycoprotein levels in human immunodeficiency virus-infected subjects on antiretroviral regimens. Drug Metab. Dispos. 30:859–860
Hayton W.L., (2000). Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci. 2:E3
Wrighton S.A., Brian W.R., Sari M.A., Iwasaki M., Guengerich F.P., Raucy J.L., Molowa D.T., Vandenbranden M.,(1990). Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol. Pharmacol. 38:207–213
Sung C.Y., Way E.L., (1953). The metabolic fate of the optical isomers of methadone. J. Pharmacol. Exp. Ther. 109:244–254
Levitt D.G., Schnider T.W., (2005). Human physiologically based pharmacokinetic model for propofol. BMC Anesthesiol. 5:4
Farrar D., Allen B., Crump K., Shipp A., (1989). Evaluation of uncertainty in input parameters to pharmacokinetic models and the resulting uncertainty in output. Toxicol. Lett. 49:371–385
Loimer N., Schmid R., (1992). The use of plasma levels to optimize methadone treatment. Drug Alcohol Depen. 30:241–246
Sprenger K.B., Huber K., Kratz W., Henze E., (1987). Nomograms for the of patient’s plasma volume in plasma exchange therapy from height, weight, and J. Clin. Apher. 3:185–190
Linderkamp O., Versmold H.T., Riegel K.P., Betke K., (1977). Estimation and of blood volume in infants and children. Eur. J. Pediatr. 125:227–234
Gray D.S., Fujioka K., (1991). Use of relative weight and Body Mass Index for the determination of adiposity. J. Clin. Epidemiol. 44:545–550
Clarys J.P., Martin A.D., Drinkwater D.T., (1984). Gross tissue weights in the human body by cadaver dissection. Hum. Biol. 56:459–473
Ogiu N., Nakamura Y., Ijiri I., Hiraiwa K., Ogiu T., (1997). A statistical analysis of the internal organ weights of normal Japanese people. Health. Phys. 72:368–383
Bailey B.J., Briars G.L., (1996). Estimating the surface area of the human body. Stat. Med. 15:1325–1332
Noda T., Todani T., Watanabe Y., Yamamoto S., (1997). Liver volume in children measured by computed tomography. Pediatr. Radiol. 27:250–252
Watanabe Y., Todani T., Noda T., Yamamoto S., (1997). Standard splenic volume in children and young adults measured from CT images. Surg. Today 27:726–728
Kasiske B.L., Umen A.J., (1986). The influence of age, sex, race, and body habitus on kidney weight in humans. Arch Pathol. Lab. Med. 110:55–60
Dekaban A.S., (1978). Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann. Neurol. 4:345–356
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, F., Tong, X., McCarver, D.G. et al. Population-Based Analysis of Methadone Distribution and Metabolism Using an Age-Dependent Physiologically Based Pharmacokinetic Model. J Pharmacokinet Pharmacodyn 33, 485–518 (2006). https://doi.org/10.1007/s10928-006-9018-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-006-9018-0