Abstract
Hydrogels are three-dimensional cross-linked stable network insoluble in water, which gives them a remarkable capacity to absorb both water and biological fluids. Hydrogel has been synthesized from natural or synthetic polymers and/or monomers, which have made tremendous advancements in many different applications. Composite hydrogel is a type of hydrogel prepared by grafting hydrophilic groups, such as hydroxyl (–OH), carboxylic acid (–COOH), imide (–CONH), sulfonic acid (–SO3H), amine (–NH2), and amide (–CONH2), into the polymer chain’s backbone and adding some additives such as kaolin, zeolite, or even different types of nanoparticles. Whereas the polymeric composite hydrogels exhibit stimuli for different properties such as pH, temperature, or light, which may affect swelling, mechanical properties, and self-healing, which in turn play vital roles in different areas. Hence, numerous efforts have been made to synthesize polymer-based composited hydrogels via physical or chemical crosslinking techniques to enhance their physiochemical, biological, and many other properties. Many researchers are currently paying attention to hydrogels and their applications, including wastewater treatment and purification, medical and biomedical applications, agricultural applications, and many other industrial applications. The aim of this review is to summarize the classification of composite hydrogels based on their chemical and physical crosslinking techniques, in addition to the different polymers and additives used to prepare composite hydrogels. Furthermore, the impact of hydrogel on health and the environment has been discussed. Other significant issues were also presented, including the challenges that face hydrogel production and application, which have been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogel refers to a water-insoluble polymeric network with a high-water absorption capacity. Wichterle and Lim made the initial suggestion in the 1960s [1]. Additionally, the hydrogel is a network of crosslinked, chain-based macromolecular polymer gel. Hydrogels can have different 2D or 3D architectures due to chemical or physical cross-linking [2, 3].
Due to their high strength-to-density ratio, processing simplicity, and comparatively low manufacturing energy requirements, polymers have experienced the biggest development in uses for hydrogel synthesis during the past few decades [4]. However, most polymers are neither useful nor strong enough mechanically, particularly in terms of electrical and thermal conductivity [5]. More than 95% of polymers are compounded in manufacturing with organic or inorganic additives to generate polymer-based composites, which are designed to get around these constraints. In the first generation of hydrogels, they were often made of a single polymer and frequently had a single purpose [6]. While, after a period of time new hydrogels composed of more than one polymer and monomer that which called composite hydrogels have been synthesized. This composite hydrogel can display a variety of properties because it is made up of individual components. Where, by combining various polymers, composite hydrogels can carry out different functions [2, 7]. A composite polymer hydrogel is created using various neutral, cationic, and anionic monomers. The mechanical and other different properties of composite hydrogels as well as their applications have been investigated, and several excellent reviews have been published [8].
Natural polymer-based hydrogels offer benefits as core materials used in the hydrogel production due to their biocompatibility, biodegradability, tunability, molecular binding capacity, and bioactive characteristics [9, 10].An Example of natural polymers used in the hydrogel synthesis are the polysaccharides, which are found in nature. Polysaccharides are a source of hydrogel production. Alginate, collagen, and gelatin, for instance, have all been utilized in the manufacture of biomaterials [11]. Synthetic polymers have great water absorption and excellent mechanical capabilities. Synthetic materials used in the preparation of various types of hydrogels include poly(ethylene) glycol (PEG), polycaprolactone (PCL), and polylactic acid (PLA) [6]. Because of their biodegradability and potential toxicity, polymer hydrogels have been limited in their application for various sustainability and safety concerns, making natural-source hydrogels more advantageous.
Many types of monomers have been used in the hydrogel synthesis such as acrylic acid (AA) and 2-acrylamide-2-methyl-1-propanesulfonic acid (AMPS) have been used to create different hydrogels [12]. As they all have the advantage of conspicuous film-formability, a high swelling rate, sustainability, and biocompatibility, they all exhibit different swelling behaviors in response to external stimuli such as pH [13], ionic strength, molecular species, swelling medium composition, temperature, and pressure environment. They are produced from hydrophilic monomers using either chain growth polymerization (ex. free radical polymerization) or step growth polymerization processes (ex. condensation and addition polymerization), along with a useful crosslinker to aid in the development of networks. By using molecular entanglements or chemical crosslinking, homopolymers or copolymers, whether synthetic or natural, are employed to create three-dimensional networks. Natural polymer hydrogels and synthetic polymer hydrogels can be distinguished according to the substance used to synthesize the hydrogels [14].
Hydrogel can be used in a variety of industries, including slow-release fertilizers [15, 16], soil amendments [17], drug delivery systems [18], tissue engineering [6], wound dressings, personal hygiene products [16], cosmetics, textiles, and wastewater treatment [19]. The current review provides a broad discussion and analysis of advanced hydrogel preparation and application status aspects, including hydrogel classifications, polymer sources, the most advanced and new applications, and some economics of hydrogel production [20].
Classifications of Composite Hydrogel
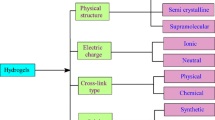
Hydrogels are often classified according to their configuration, physical appearance, polymer composition, method of crosslinking, electrical charge of the network, and sources. Figure 1 summarizes how hydrogels are classified.
For example, according to the hydrogel source, hydrogels are either natural, synthetic, or hybrid, depending on the source of the polymer network [21]. These polymer hydrogels are crosslinked as copolymeric and multi-polymeric networks [22]. Additionally, the single, double, and multi-polymers are crosslinked either chemically or physically, resulting in chemical and physical hydrogels that are single, double, and multi-polymers [14].
Hydrogels can also be divided into crystalline, semi-crystalline, and non-crystalline types based on their arrangement, physical structure, and chemical makeup. Importantly, crystalline and amorphous materials are both parts of semi-crystalline materials [11].
Classification According to Parent Chain Sources
Hydrophilic monomers that form double bonds during polymerization are used to create hydrogel from a polymer substrate. Natural, synthetic, or a combination of natural and synthetic polymers make up polymeric hydrogels. Different monomer types offer the hydrogel with various mechanical, salt-tolerance, and water-retention qualities [22].
Natural Polymers
Starch (St)
Starch (St) is a natural polysaccharide made up of anhydro glucose units that are linked together. Each of these units has three hydroxyl groups. Starch granules contain amylose and amylopectin [23].
Chitosan (CS)
Chitosan (CS) is a linear polysaccharide composed of -(1-4)-linked 2-amino-2-deoxy-d-glucose (d-glucosamine) and 2-acetamido-2-deoxy-d-glucose (N-acetyl-d-glucosamine) units. It is biocompatible and biodegradable, and its degradation products are non-toxic [24,25,26].
Alginate (Alg)
Alginate (Alg) is a linear polysaccharide synthesized using brown algae in addition to some bacteria. It is composed of 4-linked d-mannuronic acid (M) and its 5-epimer, 4-linked R-Lguluronic acid (G) [27].
Collagen
The major extracellular matrix protein, collagen, is found in the skin, ligaments, tendons, blood vessels, bones, and cartilage, among other connective tissues in the body. The three most prevalent forms of collagen, types I, II, and III, are among the many that have been found and characterized. Due to its significant presence in live human connective tissues and ease of extraction and purification from the tissue, type I collagen is the most significant and widely utilized kind of collagen in the preparation of hydrogels [28].
Cellulose
The most widely used, affordable, and biodegradable substance is cellulose, which is generated in large quantities from various agricultural wastes, as indicated in Fig. 2. The primary component of plants and natural fibers like linen and cotton is cellulose, a polymer of glucose. The 1,4-glycosidic connections that connect the glucose units are what cause natural cellulose's high crystallinity and insolubility in water and other common solvents. Native cellulose may be dissolved in solvents like N-methylmorpholine-N-oxide monohydrate or ionic liquids to create hydrogels, which can then be coagulated with water [16].
Sources of cellulose for hydrogel production [29]
Hyaluronic Acid (HA)
N-acetylglucosamine and glucuronic acid make up the widely distributed linear polysaccharide known as HA, which is present in connective tissue [30]. A naturally occurring biopolymer, hyaluronic acid serves a variety of purposes in the body, including wound healing, cell migration, and cell signaling. Hyaluronic acid has played a significant role in biomedical research because of its adaptability and has been used in a variety of sectors, including tissue engineering and the treatment of cancer, through a variety of various forms. It has a high viscoelasticity, is biocompatible, and doesn't cause an immune response [31].
A list of some trustworthy natural polymers utilized in the manufacture of hydrogels is shown in Table 1.
Synthetic Polymers
The most well-known synthetic and biodegradable polymers utilized in the production of hydrogels are those containing poly-hydroxyl acids, such as Polyethylene glycol (PEG), poly(glycolide-co-lactide), polyvinyl alcohol, poly(n-isopropylacrylamide), poly(l-lactic acid), and poly(l-lactide). These polymers eventually disintegrate into the respective hydroxyl acids as harmless degradation products by hydrolytic breakage of their ester linkages [14].
Poly(ethylene glycol) (PEG)
For the hydrogel crosslinking process, PEG is easily reacted with functional groups such as acrylate, thiol, epoxy, carboxylic acid, and amine. The functionalized end groups in PEG precursors can help the hydrogels' biodegradation even when the PEG backbone lacks a biodegradable component. Hydrogels used in medical applications have already been improved in terms of their biocompatibility and mechanical integrity using PEG hydrogels [41].
PVA
Due to its permeability and rubber-elastic physical characteristics (compressive, shear, and tensile modulus), as well as the high-water content, Polyvinyl alcohol (PVA), a hydrophilic polymer, has been utilized to create hydrogels for the replacement of injured cartilage [42].
Classification According to Crosslinking
Chemically Crosslinking
By combining chemical crosslinkers with polymers and creating covalent bonds, chemically crosslinked hydrogels are created. Since it does not dissolve in water without rupturing covalent bonds, this type of hydrogel is stable and long-lasting [43]. A completely cross-linked hydrogel has less design flexibility since it is challenging to decouple elements like gelation time, inward network pore size, functionalization, and degradation time. The different types of chemical cross-links and polymerization techniques are shown in Fig. 3.
Unexpectedly, by combining physical and chemical crosslinking network creation through electrostatic contact, a novel dual-network hydrogel is produced. Keep in mind that free radical polymerization may be used to create polymer hydrogels from synthetic, semi-synthetic, and natural hydrophilic polymers [44].
Chain Growth Polymerization “Free Radical Polymerization”
The best technique for making hydrogels based on different monomers, such as acrylates, amides, and vinyl lactams, is free radical polymerization. Additionally, it may be utilized to create hydrogels made from natural polymers that contain the right functional groups or have been functionalized with radically polymerizable groups. Radical polymerizations are fast and relatively impervious to impurities; they can be performed as bulk, solution (also in water), emulsion, suspension, or precipitation polymerization [11].
A series of experiments have been conducted to demonstrate the free radical polymerization process. In general, there are three stages: (1) initiation, in which initiators decompose at high temperatures and produce free radicals; (2) propagation, in which active sites attack vinyl monomers to copolymerize and complete the grafting polymerization; and (3) termination, in which a 3D network structure is created with the help of cross-linkers [12]. It should be noted that in the reaction systems, monomer cross-linking, and self-polymerization can both occur at the same time. For instance, ethylene glycol di-methacrylate (EGDMA) was used as a crosslinking agent, and benzoyl peroxide was used as a reaction initiator to create PVA-based hydrogels by chemical crosslinking with methacrylic acid in aqueous solutions [45].
Polymer hydrogels have been made from vinyl monomers like N-vinyl indole derivatives, N-vinyl pyrrolidone, and N-vinyl formamide through a process called controlled radical polymerization [46].
For the crosslinking to begin when light is applied during photo-polymerization, photo initiators must be introduced to the polymer solution. In contrast, catalyst-based free radical polymerization causes the generation of free radicals and then polymerization to crosslink the network. Examples of such catalysts are ammonium persulfate (APS) and tetramethylethylenediamine (TEMED) [11].
According to the above-mentioned method, different initiators and monomers are poured into the aqueous solution. Also, covalent bonds can be constructed between vinyl monomers. Many published articles related to superabsorbent hydrogels are based on using hydrophilic monomers. Free radicals can be generated to initiate the polymerization reaction by chemical induction systems, for instance, ultraviolet (UV) photopolymerization, thermal initiators (e.g., ammonium persulfate and benzoyl peroxide), redox-induced systems (Na2SO3 and bisulfite), and cerium nitrate [40].
Step Growth Polymerization
Condensation polymerization TEMED and N-3-dimethylaminopropyl-N-ethyl carbodiimide (EDC), two polyfunctional crosslinking agents, are gradually mixed during condensation and addition polymerization [47]. In condensation polymerization, a water molecule is released as a result of the bonds formed between the polymers. Polyesters and polyamides may be synthesized using condensation polymerization, and polymer hydrogels can also be synthesized through similar processes. To create composite polymer hydrogels, different polymers (natural or synthetic) can be crosslinked using one of the following crosslinkers: 1,6-hexamethylene diisocyanate, divinyl sulfone, or 1,6-hexane dibromide [48].
Solution Polymerization The reactants are dissolved in water at concentrations between 10 and 70% during the solution polymerization process. Before or after the polymerization, groups like carboxylic acid in the product are partly neutralized. In this process, the initiation is often accomplished chemically with a free-radical azo or peroxide thermal dissociative species or by the interaction of a reducing agent with an oxidizing agent (redox system) [43]. However, this approach is preferable at both laboratory and industrial scales because of its quick process, lower cost, and acceptable swelling qualities. A model for the solution polymerization reactor is shown in Fig. 4.
A model for solution polymerization reactor [43]
Irradiation Polymerization
Another technique used to synthesize polymer-based hydrogel is the irradiation polymerization technique. Hydroxyl groups detach from polymeric chains when aqueous solutions of polymers are subjected to gamma or electron beam radiation. These -OH groups can then attack polymer chains, resulting in the formation of more micro-radicals. As these micro-radicals mix on various polymer chains, covalent linkages are produced in a crosslinked form [2, 49].
Polysaccharides, PVA, PEG, and PAA were discovered to be polymers for irradiation-based crosslinking. It was discovered that during irradiation, polysaccharides with carboxylic acid groups participated in a crosslinking process [50]. For example, a grafted copolymer derived from gum ghatti (Gg) and acrylic acid was synthesized by gamma radiation [51]. In order to create composite polymer hydrogels, a grafting process was carried out in the presence of a low concentration of potassium persulfate initiator and MBA crosslinker using microwave (MW) or ultraviolet (UV) irradiation techniques [52]. In another study, branched polyethyleneimine (PEI)-grafted GO-AgNPs antibacterial nanocomposites (PEI-GO-AgNPs) were integrated using microwave irradiation to develop a slow-release antibacterial hydrogel for a more stable and sustained antibacterial effect. An antibacterial mechanism has been proposed according to the experimental observation [53].
Physically Crosslinking
Physically crosslinked polymer hydrogels are formed through non-covalent crosslinks [11]. This is usually achieved via diverse physical processes such as complexation (e.g., ion–polymer and polymer–polymer), hydrophobic organization, chain aggregation, crystallization, and hydrogen bonding as illustrated in Table 2. Amphiphilic block and graft copolymers assemble their chains in such a way that ordered structures are built through the aggregation of hydrophobic segments of the polymers. Because of hydrophobic interactions, isopropyl groups (for example, in poly(N-isopropyl acrylamide)) can be physically crosslinked. Physically crosslinked polymer hydrogels can also be made from polysaccharides such as dextran, chitosan, and carboxymethyl curdlan via hydrophobic organization [50].
Table 2 illustrate different polysaccharides used as substrate for the polymer hydrogel synthesis via physical crosslinking mechanism [50].
Ionic Interactions
Hydrogels can be crosslinked via ionic interactions under mild conditions at physiological pH and room temperature. Ionic groups in the polymer are not required for this cross-linking reaction. Stronger hydrogel is produced by metallic ions. An excellent illustration of a polymer that may be crosslinked by ionic interactions is alginate. Alginate is a naturally occurring polysaccharide that is crosslinked by calcium or sodium ions and yet contains mannuronic and glucuronic acids [57] (Fig. 5).
Ionic interaction [29]
Freezing/Thawing
Crystallization involves freezing/thawing process and creates a strong and highly elastic gel. For example, PVA hydrogels can be synthesized by physically crosslinking through repeated freezing/thawing methods [58].
Hydrogen Bonding
Like the development of a hydrogel based on gelatin, hydrogen bonding between polymer chains can also contribute to hydrogel formation. An electron-poor hydrogen atom and a functional group with a high electronegativity combine to form a hydrogen bond. This method of making hydrogels longer is affected by a number of factors, such as the concentration of polymers, the molar ratio of each polymer, the type of solvent, the temperature of the solution, and the degree to which polymer functions are linked [29, 58] (Fig. 6).
H-bonding [29]
Based Polymer for Composites Hydrogel
Depending on the base polymers used to make the hydrogel substrate, different types of functional groups like carboxylic acid, amine, hydroxyl, amidoxime, and sulfonic acid groups can be added. This can improve the ability to absorb and many other properties [11].
For a variety of uses, hydrogel-composite preparations have recently attracted a lot of attention. There are numerous drawbacks to synthetic hydrogels, including their high production costs and latent toxicity [14]. Researchers have created hydrogel composite materials made of natural, inexpensive, nontoxic, and biodegradable components to address these main limitations relating to cost and potential environmental issues. For instance, combining the hydrogel with a substance that has a greater adsorption ability may dramatically increase the sorption capacity [59]. For example, the different ways to fabricate CS-based composite hydrogels are:
-
(a)
Solvent evaporation method: CS is mixed with acetic acid and spread on a plate, where it is kept drying at an elevated temperature (around 65 °C) [60].
-
(b)
Neutralization method: by adding CS solution through a dropper into a dilute NaOH solution, beads are obtained. This can be done in a water–ethanol mixture, as chlorine does not dissolve in ethanol. The formed beads might then be subjected to crosslinking [61].
-
(c)
Crosslinking method: Membranes can be prepared by spreading CS dissolved in acetic acid on a surface and then crosslinking it with suitable crosslinking solutions such as Glutaraldehyde or Genipin. CS beads can be prepared by pouring drops of crosslinked chitosan solution into a coagulating agent such as NaOH or in a non-aqueous medium such as mineral oil [62].
-
(d)
Ionotropic gelation method: The preparation can be accomplished by adding polyanions such as xanthan, alginate, or carrageenan into a cationic CS solution [63].
-
(e)
Freeze-drying method: Hydrogels are used for tissue engineering and regenerative medicine applications and are produced by freezing CS solutions at extremely low temperatures and drying them in vacuum ovens [64]. Composite hydrogels are used for tissue engineering and regenerative medicine applications and are produced by the freeze-drying method [65].
2D GO macromolecules were used to create a PAm composite hydrogel. The inclusion of GO enhances the hydrogel's mechanical characteristics [50, 66]. In another study, a composite hydrogel composed of PVA/GO was prepared and used to remove various types of dyes, as shown in Fig. 7 [67].
Preparation of graphene embedded PVA hydrogel and their dye removal profile [67]
Typical Examples of Composite Hydrogel
Starch, chitosan, and alginate were used as the substrates in composite polymer hydrogel, which was then grafted with acrylamide using KPS and MBA as the initiator and crosslinker, respectively [52]. The hydrogel generated (PsB-g-Am) has the following grafting rate equation: Rg is equal to k[Am] [PsB]0.5 [KPS]0.5.In the used concentration range, the grafting rate is first-order dependent on the starting concentration of [Am] and square root dependent on the starting concentrations of [PsB] and [KPS].
The swelling of the chitosan-grafted acrylamide/acrylic acid hydrogel has been studied. According to statistics, swelling increases with temperature up to 60 °C before beginning to decrease as the temperature rises higher [68].
The swelling degree gradually increased after soaking the hydrogel in distilled water, and the swelling degree kinetic studies of the hydrogels were conducted at the first 8 h, where the hydrogels' swelling degrees at the three different temperatures are very high, as seen in Fig. 8. Swelling values of 1.4, 1.5, 1.4, 2.3, and 0.7 g of water per g of dry hydrogel were observed after 8 h at RT, 30, 40, 60, and 80 degrees Celsius, respectively. As seen in Fig. 8, the degree of hydrogel swelling increased as the temperature increased. At 60 degrees Celsius, swelling is twice as large as it is at room temperature [68].
Swelling degree in terms of water absorbed per dry hydrogel as a function of time and temperature [68]
In another study, a novel composite hydrogel adsorbent has been developed. The composite hydrogel is composed of zeolite and crosslinked chitosan and has been applied to remove acid red 88 dye from an aqueous solution [69].
Cellulose-based acrylic acid hydrogel has been created using rice straw as the starting point. The heterogeneous reaction was used to create the cellulose-based acrylic acid hydrogel, which has a swelling ratio greater than 300 percent [70]. For enhancing maize production in salt-affected soil as well as in the growth promoters of maize under water stress, a comparison between the rice straw-based hydrogel and the commercially available acrylamide hydrogel was explored. In general, the inclusion of hydrogel improves the efficiency of using water and nutrients, as well as nutritional concentration and absorption.
The microwave (MW) and ultraviolet (UV) irradiation have been used to graft acrylamide onto alginate and chitosan. Reverse osmosis brine and salty solutions were softened using prepared polysaccharide hydrogels. Alginate and chitosan hydrogels that were created were characterised (Alg-UV and Alg-MW). It was shown that swelling increased 16.7 to 21 times more in distilled water than in saline solutions. For grafted acrylamide hydrogels with Alg-UV and CS-MW, respectively, the maximum obtained swelling ratios in distilled water were 168 and 173 g/g. Maximum calcium absorption capabilities of Alg-MW and UV-prepared alginate and chitosan hydrogels were 54 and 34 mg/g from seawater for both dry and pre-swollen hydrogels, respectively. Using dry alginate hydrogels and chitosan hydrogels, respectively, the maximum magnesium adsorption capabilities from brine were 280 and 316 mg/g [71].
Applying the free radical polymerizing acrylic acid (AA) in the presence of sodium alginate, a new nanocomposite hydrogel (NCS) has been created, which was then loaded with Cu2+ ions and subjected to an ammonia reaction. The produced hydrogel was used in the dye removal process. At a concentration of 10 mg/L and a pH of 6, it was discovered that crystal violet (CV) and malachite green (MG) had removal efficiencies of more than 96 percent. Both the Freundlich isotherm and the pseudo-second-order kinetic models are effective in describing the dye adsorption on the NCS. The reusability tests revealed that after eight cycles, around 95% of the initial adsorption was achieved [38].
The swelling of hydrogel and NCS in the distilled water has been investigated (Fig. 9). The equilibrium swellings of the hydrogel and NCS were found to be 1134 g/g and 256 g/g, respectively.
Swelling behavior of the hydrogel and NCS (a) comparison of the hydrogel and NCS for adsorption of CV (b) (T = 25 °C, the adsorbent dosage = 0.3 g/L, pH 6, Ci = 10 mg/L, t = 2 h) [38]
Tetra-ammine copper (II) sulphate nanoparticles' interaction with the hydrogel's carboxylate groups may be the cause. As seen in Fig. 3, tetra-ammine copper (II) sulphate can function as a cross-linker and reduce swelling capacity. The hydrogel's ability to remove dye is considerably enhanced by the addition of the nanocomplex. However, the nanoparticles may also interact with the dye molecule to speed up dye adsorption.
Using cost-effective modified adsorbents, carried out batch adsorption tests for the adsorption of heavy metals. Microwave (M) irradiation method (ACM) or ultrasonic (U) irradiation technique have been used to create polyacrylate hydrogels (ACU). As shown in Fig. 10, the results of equilibrium adsorption experiments showed that the Langmuir isotherm model is better fitted than any other model, with maximum adsorption capacities of 84.5, 73.3, 61, and 97 mg/g for Cr, Co, Ni, and Pb, respectively. For all ions other than Ni, the Tempkin isotherm yielded equivalent results. The pseudo-second-order kinetic model provided the most accurate description of the adsorption kinetics of Cr, Co, Ni, and Pb using all the composite hydrogels. Investigations were also conducted on further mixtures of acrylic acid salts with Egyptian kaolin (AKM and AKU) or zeolite (AZM and AZU) [72].
Adsorption capacities of the composite hydrogels for different heavy metals (Ci = 10 mg/L, pH = 4) [73]
Figure 10 makes it evident that adding kaolin and zeolite to the polyacrylate hydrogel structure boosted Cr absorption (by more than 80%), notably for samples of hydrogel that had been made using a microwave, while also marginally increasing Co and Ni uptake. Additionally, Pb uptake increased for samples treated by microwave and ultrasonic using zeolite (27% and 3%, respectively).
For the chosen adsorbent, adsorption kinetics were investigated from 10 min to 24 h. In general, the metals adsorbed rise quickly in the first hour and continue to rise for 3 h for Pb and 5 h for Cr, Co, and Ni, after which the adsorption capacity increases by no more than 2 to 5%.
This pattern could be described by the initial quick absorption of metal ions and subsequent sluggish kinetics into the remaining empty sites, where repulsive forces from the initial adsorbed layer tend to reduce metal removal on the open sites [54]. The ideal contact period is therefore set at 3 h for Pb and 5 h for the other ions.
Composite Hydrogel Containing Nanoparticles
Due to their unique features compared to bulk materials, nanoparticles are being used in everyday consumer goods and appliances. Public discussion about the safety of nanoparticle technology has been sparked by this trend, and regulatory bodies have stepped in in several nations. By incorporating nanoparticles into hydrogels, it may be possible to overcome application problems while lowering environmental and human health hazards. Additionally, it was believed that the creative fusion of these two very different sorts of materials would produce a variety of property improvements in addition to structural diversity. The main goal of research on hydrogel-nanoparticle composite materials was to improve these properties, which led to better mechanical strength and response to stimuli [74].
Synthetic polymers are renowned for their controlled structures and intended mechanical qualities. Nanoparticle-encrusted polymer hydrogels have been created with appealing characteristics. The polymer hydrogels' surface area and pore diameters are altered by highly active nanoparticles, which improves the adsorption efficiency even further. Additionally, compared to their pure forms, the mechanical and thermal characteristics of nanocomposite-embedded polymer hydrogels were altered by a potent synergistic interaction between the nanofillers and polymer matrix [8]. To provide plenty of active sites for adsorption and cross-diffusion pathways for adsorbate molecules, a range of nanoparticles, including TiO2, ZnO, Fe-nano, and Ag-nano, were investigated in this respect [75].
There are different sources for NP composite hydrogel preparation. Polymers are both natural and synthetic. This type of nano-composite hydrogel has many applications such as biosensing and environmental utilities, which are summarized in Fig. 11.
Schematic representation of Nano Composite Hydrogel based on the source of origin and application in nanotechnology [62]
PVA-Based Nano-hydrogel
It has been reported that PVA polymer-based nano-hydrogels and modified PVA hydrogels employing functional components and nanoparticles have good stimuli-responsive biocompatibility in a variety of applications [62].
PVA/CS/nano-zinc oxide nanocomposite hydrogels were created by the freeze-thaw method and used as wound dressings. The findings showed that longer freeze-thaw cycles decreased pore size, increased porosity, and enhanced wound fluid absorption. Lowering the reaction temperature increased the elastic modulus and tensile strength while decreasing the elongation at break. The biocompatibility, antibacterial properties, ability to effectively cure wounds, and toxicity of the developed HG were all tested [76].
To create an antibacterial hydrogel, silver nanoparticles (AgNPs) have been added to a PVA/bacterial cellulose (BC). As seen in Fig. 12, the hydrogels had excellent antibacterial qualities and good biocompatibilities, in contrast to the PVA/BC hydrogel's lack of antibacterial activity [41].
Composite hydrogel grown on agar plates after incubation with Escherichia coli and Staphylococcus aureus [41]
Alginate-Based Nanohydrogel
Alginate-based nano-hydrogels, which are nanomaterials made of gels, have been recognized for their use in regenerative medicine and antibacterial and antimicrobial applications [77]. Alginate has a significant function as a prodrug and has previously been investigated for its critical role in biomedical applications due to its non-toxicity, biocompatibility, and pH sensitivity. Gum acacia copolymer-based alginate-based nanohydrogels have been developed for their versatile and efficient antibacterial activity. [62].
Hyaluronic-Based Nanohydrogel
Due to its special characteristics and utilization in drug delivery systems, HA-based nanohydrogel has demonstrated considerable potential for nanotherapeutics and nanomedicine [78]. Ketal crosslinkers used the surfactant-free one-pot approach to create hyaluronic-based nanohydrogels [62].
Reaction Rate for Polymer Composite Hydrogel
The main task of all studying the reaction rate or in more specified words is to study the kinetic experiments is to assess the grafting rate via a variety of experimental approaches. The experiments carried out to determine the kinetics rate coefficients could be divided into two areas. The first approach centers on the accurate measurement of the overall polymerization rate, whereas the second one concentrates on the analysis of the resulting molecular weight distributions. If all the rate coefficients for a polymerizing system are known, it is possible to predict the kinetics of the overall polymerization process, including the full molecular weight distributions.
Several authors studied the kinetics of graft polymerization of monomers onto polymer [68, 79]
The rate of graft polymerization (Rg) is estimated by the following equation:
where Mm represents monomer molecular weight (g/g mol).
The rate of the graft polymerization (Rg) (mol/l s) depends on the concentrations of polymer, monomer and initiator as follows:
where k is the graft rate constant, P, M and I denotes for polymer, monomer and initiator, respectively: a, b and c are constants.
A summary of reaction rate equation for grating different vinyl monomers onto polysaccharide chains monomer has been illustrated in Table 3
Super Absorbent Polymer Composite Hydrogel
By cross-linking copolymerization with different synthetic monomers, such as vinyl monomers like NIPAM, sulfo-propyl methacrylate, potassium salt, and potassium acrylate, as well as with natural polymers, hydrogels with high absorption capacities, or superabsorbent hydrogels (SAPs), can be created (i.e., carboxymethyl cellulose and gelatinized corn starch). The main substance utilized in the production of superabsorbent hydrogels is polyacrylate. By adding linked pores to the polymer network, "super-porous" hydrogels with quick swelling kinetics and superabsorbent characteristics might be created. When these hydrogels are in the xerogel state, the pore size is hundreds of micrometers. The superabsorbent hydrogels poly(N-isopropylacrylamide/acrylic acid), or P(NIPAM/AA), were created using the free radical polymerization process in the presence of the appropriate cross-linker agents, initiators, and solvents [82].
In reaction to certain external stimuli like temperature, solvent quality, pH, electric field, etc., hydrogels may show abrupt volume changes [83]. Superabsorbent hydrogels have many various applications such as biomedical applications, wastewater treatment and agricultural applications [84]. When taking agricultural application as an example of using superabsorbent hydrogels it can found that when using the right amendment and different doses of soil to hydrogel ratio, the addition of hydrogel polymer can boost soil's ability to retain water by 50–70%. Soil bulk density can decrease by 8–10% over time. So, super absorbent polymer hydrogels have many advantages when applied in the agricultural flied and these advantages may be summarized as:
-
Less affected by salts
-
Exhibits maximum absorbency @ temperatures (40–50 °C), characteristic of semi-arid and arid soils.
-
Absorbs water up to 400 times its dry weight.
-
Low rates of soil application—1–2 kg /ha for nursery horticultural crops; 2.5–5 kg/ ha for field crops
-
Reduces leaching of herbicides and fertilizers
-
Helps plants withstand prolonged moisture stress.
-
Improves seed germination and seedling emergence rate.
-
Reduces nursery establishment period.
-
Reduces irrigation and fertigation requirements of crops.
-
Promotes early and dense flowering and fruiting/ tillering.
-
Delays onset of permanent wilting point
Conductive Composite Hydrogel
Electrical current is conducted across polymer chains through p-electron conjugation in conductive hydrogels, a subcategory of hydrogel made up of conductive polymers. Here, the conducting polymer's atoms are linked together by conjugated unsaturated bonding, which leads to the delocalization of electrons along the polymer's conjugated bond. A number of dopants may be easily and affordably incorporated into conducting polymers to significantly increase their conductivity. As shown in Figure 13, polyaniline (PANI), polypyrrole (PPy), and polythiophene (PTh) are a few examples of significant conjugated conducting polymers [1].
These conducting polymers have electrical conductivities between 10−6 and 10−1 S cm−1, which is greater than insulating polymers' and lower than typical metallic conductivities. By adjusting the externally introduced dopant and its concentration, the conducting polymer's conductivity may be tailored. Additionally, because of their nanoscale-level restricted dimensions, conductive polymers display distinctive mechanical and optical characteristics [85].
Polyaniline (PANI) Based Conducting Hydrogels
PANI hydrogels are made of conductive PANI. These are frequently created by polymerizing the aniline (ANI) monomer inside a non-conductive gel matrix or by adding the conductive polymer PANI to the gel matrix. ANI-to-PANI hydrogel in-situ polymerization is commonly referred to as a “dual network gel” [86].
In situ polymerization was used to create sodium alginate-PANI (Alg-PANI) composite hydrogel for super-capacitors. The reinforced Alg-PANI hydrogel has an outstanding compressive strength of 41 kPa and a strong conductivity of 10–3 S cm−1. Because it lacks adhesive and conducting filler, Alg-PANI hydrogel is used directly as an electrode material for supercapacitors [50].prepared an adsorptive polyacrylonitrile-based hydrogel blend has been prepared to examine heavy metal removal in simulated effluents incorporating chromium and nickel. Electrical conductivity measurements indicated the presence of PANI in the polymeric blend, which assisted in the increase in conductivity of PAN hydrogel. Also, the effect of the contact time of the used hydrogel and the adsorption capacity of Ni have been studied, as shown in Fig. 14. The maximum nickel adsorption was 8 mg/g at pH 7 and 2 h of contact time [86].
Polypyrrole (PPy) Based Conducting Hydrogels
PPy-based hydrogel is another type of conductive polymer that has been applied in the synthesis of hydrogels. PPy is rigid and insoluble because of the delocalized p-electrons in the polymeric chains; therefore, the synthesis of PPy-based conductive hydrogels remains challenging.
The application of hydrogels in flexible sensors requires high mechanical toughness and excellent self-recovery to achieve large-range strain sensing and long-term cycling stability. Conductive hydrogels possess the additional property of conductivity, which endows them with advanced applications in actuating devices, biomedicine, and sensing. The conductive hydrogels used in the field of strain sensors have a conductive filler, including ionic conductors, conducting nanomaterials, and conductive polymers.
Directly doping soluble inorganic salts into hydrogel is a simple and straightforward approach to preparing conductive hydrogel. Table 4 illustrates the performance of some ionic conductive hydrogels [1].
Production Steps of Polymer Composite Hydrogel
The production of polymer composite hydrogel is composed of five main steps which are summarized as follow.
Pretreatment
For preparing the raw materials which will be used in the polymer composite hydrogel some pretreatment steps have been conducted.
In some cases, there is a process for heating the monomer before staring the reaction, Fig. 15 shows a device for use in a continuous heating technique to heat an aqueous solution of an acrylic acid-based monomer [91].
Isothermal tank for heating monomer aqueous solution [91]
Reaction Step “Polymerization”
A cross-sectional view of a mixer that may be used for a process in line with the polymer hydrogel preparation is shown schematically in Fig. 16 [92].
Reactor used for the hydrogel production [92]
Milling the Produced Hydrogel
After the completion of the hydrogel synthesis, a bulk product has been obtained. Before going further, this large piece should be cut into smaller pieces using a suitable cutting technique. Figure 17 illustrates a mill used for cutting the produced hydrogel.
A shredder mill used for cutting the produced hydrogel [83]
Filtration and Washing
Filtration is the process of removing unreacted monomers and initiator and crosslinker residues from remaining liquids.
One of the commonly used washing techniques is the washing with 75% ethanol three times is critical for removing the remaining unreacted monomers [86].
Drying
The drying step is the final step in hydrogel production. In terms of drying rate, either a conventional shelf-type drying or a tower-type (as shown in Fig. 18) drying according to this invention may be used for the hydrogel drying.
Tower dryer [93]
Hydrogel Applications
In the following section some of the main hydrogel applications will be discussed.
Agricultural Applications
The behavior of a slow-releasing hydrogel for NH4+, H2PO4−, and K+ in soil has been studied [94]. Slow-release environmental and economic problems are successfully addressed by polysaccharide-based St, which increases fertilizer consumption efficiency and decreases the frequency of water irrigation [3].
Additionally, ammonium nitrate (N source) and potassium dihydrogen phosphate (PK source) have been stored using crosslinked AA/CS hybrids because the latter is difficult to do so in its natural condition due to its explosive nature. Chitosan was combined with cellulose to reduce the acid resistance and enhance the mechanical characteristics [64].
Table 5 shows the various soil types and the recommended appropriate dosage of hydrogel that may be utilized for them [64].
The application of polymeric composite hydrogel as a soil conditioner used to improve the aggregate conditions and also to reduce soil erosion and to prevent crust formation and general stabilization. The main effect of using hydrogel in agricultural may be summarized as follows [3].
To test the soil's ability to hold water and its degradability, a new superabsorbent based poly (aspartic acid) hydrogel has been created. Results showed that after 23 days of treatment, the maximum water content was 22.6%. The microbe destroyed 47.8 wt% of the substance [95]. According to Yang et al., double-coated fertilizers could be created by mixing liquefied corn stover and isocyanate with biobased polyurethane. Chicken feather protein was infused into the hydrogel network at the same time. Corn had a 25.6% better nitrogen-utilization efficiency than urea-treated corn [96].
Soil Fixation
Hydrogel improves the stabilization and solidification of soil by varying the physical and chemical features of soil. Hydrogel can incorporate to improve water retention in the soil.
Soil Conditioning
Hydrogel aids to increase the available water content of soil to improve plant growth.
Soil Erosion Control
Wind and rain are of the most significant environmental problem for agricultural land. As a method for solving these problems, hydrogel is applied to aggregate the soil by surface treatment and hence provide surface stabilization during the early phase of crop growth.
Seepage Control
Hydrogel acts as a membrane in the soil that restricts the movement of water, thereby protecting crops from salt damage.
Biomedical Applications
Hydrogels, which have applications in medical implantable devices, robotic grippers, diagnostic tools for artificial muscles, stabilization of bone implants, intimal thickening in animals, and reducing thrombosis, mimic the behavior of human organs in response to changes in environmental conditions such as pH, temperature, enzymes, and electric fields [19,20,21,22,23,24,25, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72, 74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98].
Contact Lenses
Hard contact lenses are primarily based on hydrophobic materials such as PMMA or poly (hexa-fluor isopropyl methacrylate) (HFIM), whereas soft lenses are based on hydrogels. Soft contact lenses can be produced with different techniques, such as spin-casting, mold-casting, and lathe-cutting. A polymeric hydrogel should have a high oxygen permeability [99].
The equilibrium water content (EWC) of a hydrogel is defined as:
where m is the weight of water in polymer and mtot is the total weight of hydrated polymer EWC could change with temperature, pH, and osmolarity. For example, PHEMA contact lenses contain approximately 38–40% of water in the fully hydrated state. They typically show low variability with changes in external factors [99].
Wound Dressing
The primary commercial focus is shifting to the research and development trend of hydrogels as polymeric temporary dressing membranes. Thus, certain commercial hydrogels have entered the market as dressing materials, with brand names including "Geliperm," "Curasol," and "Tegagel." By chemically crosslinking acrylamide and methylene-bis-acrylamide with polysaccharides, these hydrogels were created [100].
As damaged tissue is placed and covered by healthy tissue, hydrogel provides a semipermeable surface that allows normal sodium discharge to occur [101].
The following explanations were used in the initial attempts by biomaterial scientists to argue that hydrogels as dressing membranes satisfy all standards for wound healing and are suitable candidates for burn victims in the quickest period possible: (1) Hydrogels regulate the body's lost liquids and fluid loss; (2) they keep the wound area moist and wettable; and (3) they have a structure and compatibility similar to tissue. The benefits listed above apply to most polymeric dressing materials, such as hydrogels, foams, films, hydrocolloids, and alginates [102].
Drug Delivery
Before placing the hydrogel-drug complex in the body, hydrogels are usually formed outside of the body and impregnated with drugs [103]. Different drugs have been loaded into the hydrogel backbone. Many factors that are controlling the release of the medication in a model of the human body. pH is the most important factor that affects how drugs are released from hydrogels [23]. Other important factors include the hydrogel's composition, geometry, manufacturing method, type of drug, and environmental conditions during the release period [104].
For solutions containing 8% by weight or volume of salt, turbidity variations of chitosan and chitosan/glycerol phosphate solutions have been observed over time. At 37 °C, the turbidity of the chitosan solution did not appreciably alter over time [22].
Chitosan hydrogels crosslinked with glutaraldehyde had 600–850% swelling values, which reduced when increasing the glutaraldehyde concentrations. Rapid hydrogel swelling (less than 20 min, for example) can lengthen the period that medications remain in the stomach and prevent early gastric emptying [105]. Additionally, the hydrogels' mesh size increases throughout the biodegradation process, allowing pharmaceuticals to diffuse out of the hydrogels [7].
Tissue Engineering
One of the main elements of extracellular matrices is chitosan (ECM). Chitosan is a perfect three-dimensional milieu used for cell encapsulation because it is biocompatible, biodegradable, hydrophilic, and non-toxic—features that are similar to those of the natural ECM. These characteristics make it a popular choice for a scaffolding material in cell-delivery treatments and tissue engineering. However, because chitosan scaffolds lack the mechanical characteristics of real bone, they are unable to support the weight of bone implants. Furthermore, chitosan's hydrogels are frequently too soft and delicate to be employed in tissue engineering applications that call for substantial load-bearing qualities since chitosan itself is not osteoconductive, preventing it from emulating the characteristics of actual bone [6, 106].
Microneedles (MNs)
As the name suggests, MNs are tiny needles that are often placed in an array to pierce the protective layer of the skin and offer a minimally invasive method for the transdermal delivery of drugs that are typically too large to be absorbed through the skin. The outermost layer of skin, known as the Stratum Corneum, is the main barrier to the delivery of transdermal medications. MNs have the ability to pierce this barrier, establishing a direct route for the effective administration of drugs [19].
Hydrogel-Forming Microneedles (HFMs), the newest type of MN, were discovered for the first time in 2012. The other MN kinds previously discussed have a different mechanism of action than HFMs, which are made of crosslinked hydrogels that can swell. Due to the hydrophilic nature of the hydrogels, which allows them to readily absorb water, HFMs will swell when applied to the skin. This makes them suitable for biological uses such ISF uptake, which is found around cells in tissue gaps, primarily in the dermis layer of the skin. ISF can be utilized as a source of biomarkers as a result, making HFMs a minimally invasive diagnostic method [103].
MXene Applications
Due to their high surface-area-to-volume ratios, high electrical conductivities (20,000 S cm−1), redox capable surface groups, chemical/structural diversities, and large surface-area-to-volume ratios, 2D MXenes with the formula Mn + 1XnTx offer a large number of promising candidates for designing conductive 2D hydrogels. To date, a number of MXene hydrogels have been created through the use of filtration, metal ions, graphene oxide, or polymers as crosslinkers, such as polyvinyl alcohol (PVA), polyacrylamide, cellulose, chitosan, poly(acrylic acid), and poly(N-isopropylacrylamide), with some success. Despite this, MXene hydrogel research is still in its infancy and faces significant obstacles [107].
As there are numerous research have been working in the synthesis and application of hydrogel in the biomedical applications, in Table 6 some of these hydrogels and their related biomedical applications have been summarized.
Separation Technology
Due to several non-biodegradable, poisonous, and carcinogenic industrial processes (particularly in the plastic, paper, cosmetic, and textile sectors), water contamination is a significant environmental issue. Adsorption is therefore viewed as an affordable and efficient way to get rid of these dyes due to its qualities, such as flexibility in the choice of an appropriate sorbent and in the production of effluents that can be recycled. As a result, there has recently been a noticeable increase in efforts to develop hydrogels with rapid adsorption and desorption rates, simple separation, regeneration, and high adsorption capacities. Figure 19 illustrates how a hydrogel works as an adsorbent to remove contaminants from water. Composite polymeric hydrogel networks with strong water absorption and pollutant immobilization properties are one of the most promising absorbing materials for water purification [67]. These composite polymeric hydrogels are regarded as advanced composite hydrogels since they may exhibit various features in terms of gel volume, swelling, and hydrophilic and hydrophobic surface properties [113].
The way hydrogels act in water purification by contaminant adsorption [114]
Metal ions, which are highly poisonous, non-biodegradable, and carcinogenic, are also regarded as water pollutants, and they have been treated using a variety of thermal, physical, chemical, electrical, and biological techniques. The literature has reported on several sorbents made from renewable resources. The results of different composite hydrogels in removing heavy metals are shown in Table 7.
Furthermore, PVA increases the mechanical characteristics, swelling capacity, and network stability of polymer hydrogel composite backbones. For instance, the synthesized hydrogel (St/PVA/PAA) adsorbed MB through hydrogen bonding and electrostatic interaction. The result was a higher maximum MB adsorption rate (417 mg g−1) than the CMC/PVA/YE hydrogel [119]. Interestingly, even after five adsorption–desorption cycles, the St/PVA/PAA hydrogel still had a 70% MB removal efficiency, demonstrating its reusability.
During the pretreat, the St readily binds the hydroxide ion (OH), increasing its hydrophilicity. Additionally, St's crystallinity is lost, and the extra ion group that is created is suitable for higher degrees of graft polymerization, increasing the adsorption capacity [120]. An overview of the outcomes for dye removal utilizing various polymeric hydrogels is shown in Table 8.
Given the great diversity among conditions of adsorption processes in other reported studies, it is difficult to compare the efficiency of any adsorbent with other adsorbents previously reported in the literature.
Biosensor Applications
Biosensors that detect and convert biological reactions into a measurable signal have gained much attention in recent years. A biosensor is a device that uses biological agents like enzymes and bacteria to detect environmental variables like pH, temperature, and contaminants like pesticides and phenols. Biosensors based on acetylcholinesterase as the recognition component are often utilized for pesticide determination. Due to their low cost and environmentally favorable makeup, polymers can also be taken into consideration for the production of the electrodes for biosensors.
Flexible strain sensors (FSSs) have proven to be a promising candidate for smart wearable devices due to their flexibility, light weight, and biocompatibility, as opposed to traditional devices fabricated from metals or semiconductors, which can only sense small deformations less than 5% strain and are accompanied by inherent rigidity, making them uncomfortable to wear.
Chaff films and membranes, which are biopolymers, can be used as surfaces to immobilize biological analytes. Figure 20 illustrates the benefits of employing chitosan-based composite hydrogels for biosensor applications [64].
A priority pollutant with the potential to irritate the skin, eyes, and respiratory system is 4-nitrophenol. The detection of 4-nitrophenol in water samples has been accomplished using zinc oxide nanoneedles grafted with CS. With a limit of 0.23 M, lower concentrations of 4-nitrophenol could be detected, and the sensor showed a broad linear response range. It was found that these needles worked well even when they were near things that could mess with them [64].
Oil Recovery
Controlling the amount of water in industrial oils and fuels is essential for preventing deterioration during storage and ensuring their intended performance.
Hydrogels are frequently injected near the wellbore to selectively seal the zones with greater permeability or fractures to reduce surplus water production and hence boost hydrocarbon production. This directs the injected water towards low-permeability, upswept hydrocarbon-rich zones [125].
Polymer hydrogels can be employed as blocking gels to alter the properties of the rock and the displacement process, improve sweep efficiency, and ultimately increase oil recovery rates. These systems generally have radial penetration of 5–15 ft (1.5–4.5 m) and are injected into the reservoir formation via an injection well or a producing well. To seal the most porous areas, the gel must flow to those areas and build an impenetrable barrier in the rock's pores. This process can cause the amount of water to go down and the amount of oil to go up [22].
The volume to be treated, the kind of base oil, and the amount of water to be removed all play major roles in determining which option is the most economically and technically feasible. For oils with a high-water content, physical unit operations including gravity separation, centrifuging, and coalescing filters are often used. However, mass transfer processes such as distillation and salt beds are frequently used technologies for processing oils with low water concentrations [23].
The water content may go beyond the solubility limit during the refining, transporting, or storing process, creating suspended and free water [11]. Water becomes a contaminant and causes phase separation when it is suspended and free. While some suspended waters could naturally split over time, others might stay suspended forever [15]. The oil and free water may transfer momentum during the pumping process, causing temperature changes and the formation of suspended water, which disperses the water in tiny droplets [23].
Environmental Assessment of Polymer Hydrogel
Many attempts have been made to construct hydrogel-based materials for medical and pharmaceutical applications to minimize the unfavorable systemic toxicity of medicines and dosage strength. The pharmacies have a wide selection of hydrogels that have received clinical approval. The primary need for using a polymeric hydrogel is that it must be biocompatible with the tissues around it. Materials that do not include cytotoxic precursors or soaking products are required for hydrogel systems. The vitality of cells and their proliferation are important metrics for the biocompatibility assessment. Only in situ hydrogels are utilized for encapsulating cells [24, 26].
At first, synthetic hydrogels couldn't be used in medicine because the unreacted monomers and cross-linkers were toxic, they didn't break down easily, and they couldn't form hydrogels in physiological conditions [25].
For their use in human applications, hydrogels must first be created with a low number of leftover reactants and undesired side products. This makes it crucial to rinse them out of synthetic hydrogels using the proper solvents before analyzing them. In polymers manufactured for industrial-scale applications, residual monomer content of up to 0.5 percent and even up to 1 percent or more is frequently seen [27].
Following the production of the poly (hydroxyethyl methacrylate/methacrylic acid) hydrogel, the remaining reactants were cleaned with distilled water [28]. The synthesis was carried out using radical polymerization and ethylene glycol dimethacrylate (EGDM) as a cross-linker at various concentrations [30]. Unreacted monomers and cross-linkers were removed from synthetic P(AA/methacrylic acid (MAA)) hydrogels with acetonitrile, while Poly(N-isopropylacrylamide) (PNIPAM) hydrogels were removed with methanol. The reactants left behind after the production of P(AA/MAA) and NIPAM-based hydrogels also demonstrated a successful polymerization process [28]. Chemical and physical approaches are used to decrease the residual reactants, and it was found that the two most popular equipment types for ensuring the removal of residuals are gas chromatography (GC) and high-performance liquid chromatography (HPLC) [126].
Hydrogel Toxicity
The level of harm that a chemical compound or specific chemical combination may do to an organism is known as its toxicity. The negative outcome might range from a small side effect to a serious threat to the patient's life or survival. Acute, subphrenic, and chronic toxicity are all possible. A single or brief encounter can have adverse consequences for an organism, which is known as acute toxicity. The capacity of a toxic agent to have effects that last longer than a year but shorter than the lifetime of the exposed organism is known as subphrenic toxicity. A substance's capacity to have negative effects over a lengthy period is known as chronic toxicity [127].
Low toxicity refers to toxicity that is substantially lower than acute toxicity, which varies depending on the drug and the medium's concentration. Toxicology is a dose-dependent characteristic, and lately, a "Drug Toxicity Index" was established, where the author offered mechanistic insights and made clinical predictions. Effects on the target can be used to gauge toxicity. Utilizing the median lethal dose (LD50) and the 50% fatal concentration, the lethal dosage of a toxin, radiation, or pathogen is calculated (LC50). Furthermore, it has proposed numerous phrases and the toxicology's lethal dose [96].
Hydrogel Regeneration and Reuse (Recycle)
One of the key components of water treatment technologies that involves managing its economy is the desorption and regeneration of adsorbents in the removal of absorbates (heavy metals, dyes, etc.). Thermal, ultrasonic, chemical, and electrochemical desorption methods are often utilized. Electrical enhancement was first introduced fundamentally and at the adsorption and desorption phases because of the improvement of chemical desorption procedures through the use of electricity in various ways [128].
Polyacrylonitrile-based hydrogel has been synthesized and determined its suitability for electrically assisted regeneration in order to increase the effectiveness of heavy metal adsorption from aqueous solutions in comparison to chemical regeneration. The results showed that electrically assisted chemical regeneration had significantly higher nickel and chromium values of 51.6 and 98.3%, respectively, demonstrating the benefit of using electrically assisted chemical regeneration to improve the application of heavy metals-adsorbing hydrogels [128].
A chelator-mimetic multi-functionalized hydrogel with a high metal adsorption efficiency (cadmium, lead, and arsenic) and great reusability has been synthesized. By using a low concentration of hydrochloric acid, the heavy metals absorbed in the hydrogel network were eluted and the hydrogel was regenerated for reuse. After five adsorption/desorption reuse cycles, a removal ratio greater than 60% was obtained [129]. By applying a similar approach, a novel hydrogel containing chitosan, acrylic acid has been developed, and an amine-functionalized nano-silica. In this study, a 1 molar solution of hydrochloric acid was used to recover Pb2+-loaded hydrogel. The hydrogel was then regenerated by filtering and washing with deionized water before being utilized for the next adsorption cycle. After three consecutive cycles, the efficiency of the regenerated hydrogel remained around 685–715 mg/g [130].
According to a different study, the determined rates of photobleaching the dye with the same photocatalyst are 0.02, 0.018, and 0.009 min−1 for the first, second, and third experiments, respectively. As a result, the TiO2 supported on the copolymer hydrogel could be used two to three times [131].
Challenges and Future Development Activities
The best hydrogel manufacturing and synthesis factories are those that are small to medium-sized. Therefore, finding a cross-linking technique that is effective, safe, and environmentally friendly is crucial for creating biocompatible hydrogels with strong mechanical capabilities.
A greater knowledge of the nature of biomaterials and the principles controlling their growth is possible thanks to the scientific and technical tools needed for the development of biomaterials in the long history of human civilization. The methodology of science and technology plays a significant role in directing the development of biomaterials from the viewpoint of the dialectics of nature. Research in science and technology has been crucial in the creation of biomaterials. Finally, the area of biomaterials has been infiltrated by nanoscience and technology, and people have gradually come to understand the enormous development potential of biomaterials, opening many prospects for relevant scientific researchers. The design and preparation of biologically functional materials has emerged as one of the most significant and active areas in the field of life science and medical research. This area has drawn increasing interest from interdisciplinary researchers and clinicians due to the ongoing, in-depth development of science and technology. It is important to not undervalue this tendency in future growth.
Composite hydrogels should consider the following factors:
-
(1)
The design of the reactor and finding a solution for gel formation with viscosity problems should be studied as an example. I may suggest "a rotary heated reactor, a steam heated reactor, a ribbon mixer with a screw around the axis, a screw mixer with four baffles, and a double ribbon mixer."
-
(2)
Enhance all mechanical characteristics to fulfil medical application criteria.
-
(3)
Another important factor to consider for the sporty treatment of pollutants is short equilibrium times or fast kinetics.
-
(4)
Make polymer hydrogels biosafe and increase their effectiveness.
-
(5)
The recovery of hydrogel from solution after the accomplishment of the adsorption (or desorption) procedure. (Conventional segregation or
-
(6)
Investigate composite modification using cutting-edge new natural functional materials hydrogels to improve performance.
-
(7)
In order to create new hydrogel materials that can be tailored to the needs of biomedical applications, it is necessary to combine new techniques, such as rapid 3D printing technology, genetic engineering, and nanotechnology. Clinical applications also call for additional experimental investigation and testing.
-
(8)
The hydrogel must be physically and chemically stable during the successive rounds of adsorption–desorption treatments (regeneration).
-
(9)
The problem of hydrogel disposal should be widely discussed.
Conclusions
In general, there are many different fields of study that fall under the umbrella of hydrogel research, and there are an increasing number of uses for hydrogels. Therefore, it is challenging to make reliable predictions about the direction of hydrogel research.
Benefits of Hydrogels that are environmentally sensitive have the capacity to recognize changes in pH, temperature, or metabolite concentration and release their load as a result. Hydrogel is more elastic and durable. Polymeric composite hydrogels have a variety of special qualities that qualify and enable them to display amazing properties, allowing them to be used as viable candidates for applications in nearly all domains, including biomedical, industrial, environmental, and agricultural areas. Significant changes are occasionally made to the field of hydrogels for their beneficial uses. The categorization of polymeric hydrogels and their most recent developments have been briefly reviewed in the current study.
References
Tang L, Wu S, Qu J, Gong L, Tang J (2020) A review of conductive hydrogel used in flexible strain sensor. Materials 13(18):3947
Zainal SH, Mohd NH, Suhaili N, Anuar FH, Lazim AM, Othaman R (2021) Preparation of cellulose-based hydrogel: a review. J Market Res 10:935–952
Liu Y, Wang J, Chen H, Cheng D (2022) Environmentally friendly hydrogel: a review of classification, preparation and application in agriculture. Sci Total Environ 846:157303
Liu X, Liu J, Lin S, Zhao X (2020) Hydrogel machines. Mater Today 36:102–124
Mondal S, Das S, Nandi AK (2020) A review on recent advances in polymer and peptide hydrogels. Soft Matter 16(6):1404–1454
Tran HD, Park KD, Ching YC, Huynh C, Nguyen DH (2020) A comprehensive review on polymeric hydrogel and its composite: matrices of choice for bone and cartilage tissue engineering. J Ind Eng Chem 89:58–82
Rodríguez-Rodríguez R, Espinosa-Andrews H, Velasquillo-Martínez C, García-Carvajal ZY (2020) Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: a review. Int J Polym Mater Polym Biomater 69(1):1–20
Peng N, Zhang X, Xu H, Liu Y (2019) Polymeric hydrogels with antimicrobial activity-a review of their progress. Biomed J Sci Tech Res 23(5):17810–17823
Wang L, Liang K, Deng L, Liu YN (2019) Protein hydrogel networks: a unique approach to heteroatom self-doped hierarchically porous carbon structures as an efficient ORR electrocatalyst in both basic and acidic conditions. Appl Catal B 246:89–99
Sinha A, Kalambate PK, Mugo SM, Kamau P, Chen J, Jain R (2019) Polymer hydrogel interfaces in electrochemical sensing strategies: a review. TrAC 118:488–501
El Sayed MM (2019) Hydrogel preparation technologies: relevance kinetics, thermodynamics and scaling up aspects. J Polym Environ 27(4):871–891
Sennakesavan G, Mostakhdemin M, Dkhar LK, Seyfoddin A, Fatihhi SJ (2020) Acrylic acid/acrylamide based hydrogels and its properties-a review. Polym Degrad Stab 180:109308
Gerami SE, Pourmadadi M, Fatoorehchi H, Yazdian F, Rashedi H, Nigjeh MN (2021) Preparation of pH-sensitive chitosan/polyvinylpyrrolidone/α-Fe2O3 nanocomposite for drug delivery application: emphasis on ameliorating restrictions. Int J Biol Macromol 173:409–420
Bharskar G (2020) A review on hydrogel. World J Pharm Pharm Sci 9(7):1288–1298
Gull N, Khan SM, Butt OM, Islam A, Shah A, Jabeen S et al (2020) Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int J Biol Macromol 162:175–187
Enawgaw H, Tesfaye T, Yilma KT, Limeneh DY (2021) Synthesis of a cellulose-Co-AMPS hydrogel for personal hygiene applications using cellulose extracted from corncobs. Gels 7(4):236
Kareem A, Dere I, Gungula T, Andrew P, Saddiq M, Adebayo F, Patrick O (2021) Synthesis and characterization of slow-release fertilizer hydrogel based on hydroxy propyl methyl cellulose, polyvinyl alcohol, glycerol and blended paper. Gels 7(4):262
Zhang HJ, Sun TL, Zhang AK, Ikura Y, Nakajima T, Nonoyama T et al (2016) Tough physical double-network hydrogels based on amphiphilic triblock copolymers. Adv Mater 28(24):4884–4890
Damiri F, Bachra Y, Bounacir C, Laaraibi A, Berrada M (2020) Synthesis and characterization of lyophilized chitosan-based hydrogels cross-linked with benzaldehyde for controlled drug release. J Chem 2020:8747639
Hu Y, Du Z, Deng X, Wang T, Yang Z, Zhou W, Wang C (2016) Dual physically cross-linked hydrogels with high stretchability, toughness, and good self-recoverability. Macromolecules 49(15):5660–5668
Gull N, Khan SM, Butt MTZ, Khalid S, Shafiq M, Islam A et al (2019) In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: a preclinical study. RSC Adv 9(53):31078–31091
Chamkouri H, Chamkouri M (2021) A review of hydrogels, their properties and applications in medicine. Am J Biomed Sci Res 11(6):485–493
Qamruzzaman M, Ahmed F, Mondal MIH (2022) An overview on starch-based sustainable hydrogels: potential applications and aspects. J Polym Environ 30(1):19–50
Sadeghi-Kiakhani M, Safapour S, Ghanbari-Adivi F (2019) Grafting of chitosan-acrylamide hybrid on the wool: characterization, reactive dyeing, antioxidant and antibacterial studies. Int J Biol Macromol 134:1170–1178
Buwalda SJ, Boere KW, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE (2014) Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release 190:254–273
Vashist A, Vashist A, Gupta YK, Ahmad S (2014) Recent advances in hydrogel-based drug delivery systems for the human body. J Mater Chem B 2(2):147–166
Shao ZJ, Huang XL, Yang F, Zhao WF, Zhou XZ, Zhao CS (2018) Engineering sodium alginate-based cross-linked beads with high removal ability of toxic metal ions and cationic dyes. Carbohyd Polym 187:85–93
Nikolić L, Ilić-Stojanović S, Nikolić V (2016) Analysis of residual reactants from synthesized poly(acrylic acid-co-methacrylic acid). In: Book of abstract of the XI Conference of Chemists, Technologists and Environmentalists of the Republic of Srpska, Teslić, Bosnia and Herzegovina, pp 18–19
Saini K (2017) Preparation method, properties and crosslinking of hydrogel: a review. PharmaTutor 5(1):27–36
Sarrigiannidis SO, Rey JM, Dobre O, González-García C, Dalby MJ, Salmeron-Sanchez M (2021) A tough act to follow: collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater Today Bio 10:100098
Collins MN, Birkinshaw C (2016) Physical properties of crosslinked hyaluronic acid hydrogels. J Mater Sci 19:3335–3343. https://doi.org/10.1007/s10856-008-3476-4
Dovedytis M, Liu ZJ, Bartlett S (2020) Hyaluronic acid and its biomedical applications: a review. Eng Regen 1:102–113
Cui C, Shao C, Meng L, Yang J (2019) High-strength, self-adhesive, and strain-sensitive chitosan/poly (acrylic acid) double-network nanocomposite hydrogels fabricated by salt-soaking strategy for flexible sensors. ACS Appl Mater Interfaces 11(42):39228–39237
Tanveer M, Farooq A, Ata S, Bibi I, Sultan M, Iqbal M et al (2021) Aluminum nanoparticles, chitosan, acrylic acid and vinyltrimethoxysilane based hybrid hydrogel as a remarkable water super-absorbent and antimicrobial activity. Surf Interfaces 25:101285
Kordjazi S, Kamyab K, Hemmatinejad N (2020) Super-hydrophilic/oleophobic chitosan/acrylamide hydrogel: an efficient water/oil separation filter. Adv Compos Hybrid Mater 3(2):167–176
Altaf F, Niazi MBK, Jahan Z, Ahmad T, Akram MA, Butt MS, Sher F (2021) Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J Polym Environ 29(1):156–174
Taghizadeh M, Mafakhery S (2001) Kinetics and mechanism of graft polymerization of acrylonitrile onto starch initiated with potassium persulfate. J Sci Iran Polym J 12:333–338
Damiri F, Kommineni N, Ebhodaghe SO, Bulusu R, Jyothi VGS, Sayed AA et al (2022) Microneedle-based natural polysaccharide for drug delivery systems (DDS): progress and challenges. Pharmaceuticals 15(2):190
Luo H, Dong F, Wang Q, Li Y, Xiong Y (2021) Construction of porous starch-based hydrogel via regulating the ratio of amylopectin/amylose for enhanced water-retention. Molecules 26(13):3999
Mozaffari T, Vanashi AK, Ghasemzadeh H (2021) Nanocomposite hydrogel based on sodium alginate, poly (acrylic acid), and tetraamminecopper (II) sulfate as an efficient dye adsorbent. Carbohyd Polym 267:118182
Zhao B, Jiang H, Lin Z, Xu S, Xie J, Zhang A (2019) Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohyd Polym 224:115022
Zhao Z, Yan R, Zharnikov M (2021) The Effect of ultraviolet light on biorepulsive hydrogel poly (ethylene glycol) films. ACS Appl Polym Mater 3(7):3446–3454
Farid E, Kamoun EA, Taha TH, El-Dissouky A, Khalil TE (2022) PVA/CMC/attapulgite clay composite hydrogel membranes for biomedical applications: factors affecting hydrogel membranes crosslinking and bio-evaluation tests. J Polym Environ 30(11):4675–4689
Ranganathan N, Joseph Bensingh R, Abdul Kader M, Nayak SK (2018) Synthesis and properties of hydrogels prepared by various polymerization reaction systems. Springer, Cham
Sikdar P, Uddin MM, Dip TM, Islam S, Hoque MS, Dhar AK, Wu S (2021) Recent advances in the synthesis of smart hydrogels. Mater Adv 14:4532–4573
Li S, Qin T, Chen T, Wang J, Zeng Q (2021) Poly (vinyl alcohol)/poly (hydroxypropyl methacrylate-co-methacrylic acid) as pH-sensitive semi-IPN hydrogels for oral insulin delivery: preparation and characterization. Iran Polym J 30(4):343–353
Ling Y, Chen L, Huang M, Zhou C, Yang L, Niu H et al (2022) A novel method for the preparation of poly (Acrylamide-co-Acrylonitrile) upper critical solution temperature thermosensitive hydrogel by the partial dehydration of acrylamide grafted polypropylene sheets. Gels 8(6):345
Maitra J, Shukla VK (2014) Cross-linking in hydrogels-a review. Am J Polym Sci 4(2):25–31
Akhtar MF, Hanif M, Ranjha NM (2016) Methods of synthesis of hydrogels—a review. Saudi Pharm J 24:554–559
Fekete T, Borsa J, Takacs E, Wojnarovits L (2017) Synthesis of carboxymethylcellulose/starch superabsorbent hydrogels by gamma-irradiation. Chem Cent J 11:46
Samaddar P, Kumar S, Kim KH (2019) Polymer hydrogels and their applications toward sorptive removal of potential aqueous pollutants. Polym Rev 59(3):418–464
Sharma SSA, Bashir S, Kasi R, Subramaniam RT (2022) The significance of graphene based composite hydrogels as smart materials: a review on the fabrication, properties, and its applications. FlatChem 100352
Wei X, Senanayake TH, Warren G, Vinogradov SV (2013) Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjug Chem 24:658–668
Sorour MH, Hani HA, Shaalan HF, El Sayed MM, El-Toukhy MA (2021) Adsorption isotherms and kinetics pertinent to modified composite hydrogel adsorbents adopted for heavy metals removal. Int J Appl Sci Eng 18(5):1–12
Yang S, Zhou Y, Zhao Y, Wang D, Luan Y (2022) Microwave synthesis of graphene oxide decorated with silver nanoparticles for slow-release antibacterial hydrogel. Mater Today Commun 31:103663
Rassu G, Salis A, Porcu EP, Giunchedi P, Roldo M, Gavini E (2016) Composite chitosan/alginate hydrogel for controlled release of deferoxamine: a system to potentially treat iron dysregulation diseases. Carbohydr Polym 2016(136):1338–1347. https://doi.org/10.1016/j.carbpol,10,048
Xiao C, Gao Y (2008) Preparation and properties of physically crosslinked sodium carboxymethylcellulose/poly(vinyl alcohol) complex hydrogels. J Appl Polym Sci 107:1568–1572. https://doi.org/10.1002/app.27203
Thivya P, Akalya S, Sinija VR (2022) A comprehensive review on cellulose-based hydrogel and its potential application in the food industry. Appl Food Res 2:100161
Pardeshi S, Damiri F, Zehravi M, Joshi R, Kapare H, Prajapati MK et al (2022) Functional thermoresponsive hydrogel molecule to material design for biomedical applications. Polymers 14(15):3126
Ullah F, Othman MBH, Javed F, Ahmad Z, Akil HM (2015) Classification, processing, and application of hydrogels: a review. Mater Sci Eng, C 57:414–433
Ahmad S, Minhas MU, Ahmad M, Sohail M, Abdullah O, Badshah SF (2018) Preparation and evaluation of skin wound healing chitosan-based hydrogel membranes. AAPS PharmSciTech 19(7):3199–3209
Kumar A, Han S (2017) PVA-based hydrogels for tissue engineering: a review. Int J Polym Mater Polym Biomater 66(4):159–182
Lakehal I, Montembault A, David L, Perrier A, Vibert R, Duclaux L, Reinert L (2019) Prilling and characterization of hydrogels and derived porous spheres from chitosan solutions with various organic acids. Int J Biol Macromol 129:68–77
Sacco P, Pedroso-Santana S, Kumar Y, Joly N, Martin P, Bocchetta P (2021) Ionotropic gelation of chitosan flat structures and potential applications. Molecules 26(3):660
Nangiaa S, Warkarb S, Katyal D (2018) A review on environmental applications of Chitosan biopolymeric hydrogel based composites. J Macromol Sci A 55(11–12):747–763
Raisi A, Asefnejad A, Shahali M, Doozandeh Z, Kamyab Moghadas B, Saber-Samandari S, Khandan A (2020) A soft tissue fabricated using a freeze-drying technique with carboxymethyl chitosan and nanoparticles for promoting effects on wound healing. J Nanoanal 7(4):262–274
Huawen H, Zhang MC, Wang M, Chen D (2017) A new insight into PAM/graphenebased adsorption of water-soluble aromatic pollutants. J Mater Sci 52:8650–8664. https://doi.org/10.1007/s10853-017-1090-x
Im K, Nguyen N, Kim S, Kong J, Kim Y, Park S, Kwon S, Yoon H (2017) Graphene-embedded hydrogel nanofibers for detection and removal of aqueous-phase dyes. ACS Appl Mater Interfaces 9:10768–10776. https://doi.org/10.1021/acsami.7b01163
El Sayed M, Yehia R, Ameen A (2021) Swelling modelling and kinetics investigation of polymer hydrogel composed of chitosan-g-(AA-AM). Egypt J Chem 64(10):5999–6005
Hidayat E, Harada H, Mitoma Y, Yonemura S, Halem HIA (2022) Rapid removal of acid red 88 by zeolite/chitosan hydrogel in aqueous solution. Polymers 14(5):893
Ibrahim MM, Abd-Eladl M, Abou-Baker NH (2015) Lignocellulosic biomass for the preparation of cellulose-based hydrogel and its use for optimizing water resources in agriculture. J Appl Polym Sci. https://doi.org/10.1002/app.42652
Sorour M, El-Sayed M, Abd El Moneem N, Talaat H, Shaalan H, El Marsafy S (2013) Free radical grafting kinetics of acrylamide onto a blend of starch/chitosan/alginate. Carbohyd Polym 98(1):460–464
Sorour MH, Tewfik SR, Abulnour AG, Shaalan HF, Hani HA, Al-Bazedi GA (2020) Development and assessment of adsorptive hydrogel: process engineering and economic considerations. Eng Rep 2(4):e12140
Wang S, Lei J, Yi X, Yuan L, Ge L, Li D, Mu C (2020) Fabrication of polypyrrole-grafted gelatin-based hydrogel with conductive, self-healing, and injectable properties. ACS Appl Polym Mater 2(7):3016–3023
Yao Y, Xia M, Wang H, Li G, Shen H, Ji G et al (2016) Preparation and evaluation of chitosan-based nanogels/gels for oral delivery of myricetin. Eur J Pharm Sci 91:144–153
Quazi MZ, Park N (2022) Nanohydrogels: advanced polymeric nanomaterials in the era of nanotechnology for robust functionalization and cumulative applications. Int J Mol Sci 23(4):1943
Khorasani T, Joorabloo A, Adeli H, Mansoori-Moghadam Z, Moghaddam A (2019) Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohyd Polym 207:542–554
Pei M, Jia X, Zhao X, Li J, Liu P (2018) Alginate-based cancer-associated, stimuli-driven and turn-on theranostic prodrug nanogel for cancer detection and treatment. Carbohyd Polym 183:131–139
Sorour MH, Hani HA, Shaalan HF, El Sayed MM, El-Sayed MM (2015) Softening of seawater and desalination brines using grafted polysaccharide hydrogels. Desalin Water Treat 55(9):2389–2397
Liming Z, Jianping G, Ruchuan T, Jiugao Y, Wei W (2003) Graft mechanism of acrylonitrile onto starch by potassium permanganate. J Appl Polym Sci 88:146–152
Taghizadeh M, Khosravy M (2003) Kinetics and mechanism of graft copolymerization of vinyl monomers (acrylamide, acrylic acid, and methacrylate) onto starch by potassium dichromate as redox initiator. Iran Polym J 12:497–505
Basu P, Saha N, Saha T, Saha P (2021) Polymeric hydrogel based systems for vaccine delivery: a review. Polymer 230:124088
Isshi K, Torii K, Torii K, Omori S, Omori S, Tanaka S, Tanaka S, Sakamoto S, Tadashi K, Kenji T, Tadashi D, Sato S (2017). Gel pulverizer, polyacrylic acid (salt) water-absorbing resin powder production method, and water-absorbing resin powder. JP 6067126 B2 2017.1.25
Sharma J, Kaith BS, Bhatti MS (2018) Fabrication of biodegradable superabsorbent using RSM design for controlled release of KNO3. J Polym Environ 26(2):518–531
Zhang Q, Liu X, Ren X, Jia F, Duan L, Gao G (2019) Nucleotide-regulated tough and rapidly self-recoverable hydrogels for highly sensitive and durable pressure and strain sensors. Chem Mater 31(15):5881–5889
El Mansoup AA, El Sayed MM, Abulnour AM, Fahmy HM, El Nashar RM (2020) Characterization and performance analysis of an adsorptive polyacrylonitrile based hydrogel for heavy metals removal. IJRTE 9(1):283–291
Yang BW, Yuan W (2019) Highly stretchable and transparent double-network hydrogel ionic conductors as flexible thermal-mechanical dual sensors and electroluminescent devices. ACS Appl Mater Interfaces 11:16765–16775
Liu J, Cao T, Ma G, Wan B (2017) Ultrasensitive wearable soft strain sensors of conductive, self-healing, and elastic hydrogels with synergistic “Soft and Hard” hybrid networks. ACS Appl Mater Interfaces 9:25559–25570
Wang Y, Zhang Y, Li S, Zhong W, Wei W (2018) Enhanced methylene blue adsorption onto activated reed-derived biochar by tannic acid. J Mol Liq 268:658–666
Hou W, Sheng N, Zhang X, Luan Z, Qi P, Lin M, Tan Y, Xia Y, Li Y, Sui K (2019) Design of injectable agar/NaCl/polyacrylamide ionic hydrogels for high performance strain sensors. Carbohydr Polym 211:322–328
Nakatsuru R (2017) Water-absorbable polyacrylic acid resin powder, and process for production thereof. EP 2 518 092 B1
Kadonaga K, Himeji JP, Kenji T, Himeji JP, Masazumi S, Seiji K (2013) Method for producing water absorbent resin. United States Patent, US 9, 796, 820 B2
Sakamoto J (2017) Porous sol gels and methods and structures related thereto. US 9, 808, 964 B2
Senna AM, Botaro VR (2017) Biodegradable hydrogel derived from cellulose acetate and EDTA as a reduction substrate of leaching NPK compound fertilizer and water retention in soil. J Control Release 260:194–201
Lu SY, Feng C, Gao CM, Wang G, Xu B, Bai X, Gao N, Liu Z (2016) Multifunctional environmental smart fertilizer based on l-aspartic acid for sustained nutrient release. J Agric Food Chem 64:4965–4974
Yong Z, Liang XY, Yang XQ, Liu HY, Yao LM (2014) An eco-friendly slow-release urea fertilizer based on waste mulberry branches for potential agriculture and horticulture applications. ACS Sustain Chem Eng 2:1871–1878
Taghizadeh M, Mehrdad A (2006) Kinetic study of graft polymerization of acrylic acid and ethyl methacrylate onto starch by ceric ammonium nitrate. Iran J Chem Chem Eng 25:1–11
Mu M, Li X, Tong A, Guo G (2019) Multi-functional chitosan-based smart hydrogels mediated biomedical application. Expert Opin Drug Deliv 16(3):239–250
Shaker LM, Al-Hamdani AH, Al-Amiery AA (2020) Vision improvement using titanium dioxide nanoparticles-doped PMMA for contact lenses. Eng Technol J 38(5):681–689
Kamoun EA, Kenawy ERS, Chen X (2017) A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 8(3):217–233
Ijaola AO, Akamo DO, Damiri F, Akisin CJ, Bamidele EA, Ajiboye EG et al (2022) Polymeric biomaterials for wound healing applications: a comprehensive review. J Biomater Sci Polym Ed 33(15):1998–2050
Ahmad F, Mushtaq B, Ahmad S, Rasheed A, Nawab Y (2023) A Novel composite of hemp fiber and alginate hydrogel for wound dressings. J Polym Environ. https://doi.org/10.1007/s10924-023-02756-7
Damiri F, Rahman MH, Zehravi M, Awaji AA, Nasrullah MZ, Gad HA et al (2022) MXene (Ti3C2Tx)-embedded nanocomposite hydrogels for biomedical applications: a review. Materials 15(5):1666
Fouad D, Bachra Y, Ayoub G, Ouaket A, Bennamara A, Knouzi N, Berrada M (2020) A novel drug delivery system based on nanoparticles of magnetite Fe3O4 embedded in an auto cross-linked chitosan. In: Chitin and chitosan-physicochemical properties and industrial applications. IntechOpen
Naahidi S, Jafari M, Logan M, Wang Y, Yuan Y (2017) Biocompatibility of hydrogel based scaffolds for tissue engineering. Appl Biotechnol Adv 35(5):530–544
Naahidi S, Jafari M, Logan M, Wang Y, Yongfang Y (2017) Biocompatibility of hydrogel based scaffolds for tissue engineering. Appl Biotechnol Adv 35(5):530–544
Li K, Zhao J, Zhussupbekova A, Shuck CE, Hughes L, Dong Y et al (2022) 4D printing of MXene hydrogels for high-efficiency pseudocapacitive energy storage. Nat Commun 13(1):1–11
Sarabahi S (2012) Recent advances in topical wound care. Indian J Plastic Surg 45(2):379
Misal RS, Potphode R, Mahajan V (2017) Review on: new approaches in self-micro-emulsifying drug delivery system. Res J Pharm Technol 10(4):1215–1224
Xinming L, Sergey, Mikhalovsky V, Susan R, Sandemanb A, Howelb L (2008) Polymeric hydrogels for novel contact lens- based ophthalmic drug delivery systems: a review. Contact Lens Anterior Eye 31(2):57–64
Paul A, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A (2014) Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 8(8):8050
Bullo S (2015) Inorganic nanolayers: structure, preparation, and biomedical applications. Int J Nanomed 10:5609
Bera R, Dey A, Chakrabarty D (2017) Tuning of the swelling and dye removal efficacy of poly (acrylamide-AMPS)-based smart hydrogel. Sep Sci Technol 52(4):743–755
Weerasundara L, Gabriele B, Figoli A, Ok YS, Bundschuh J (2021) Hydrogels: novel materials for contaminant removal in water—a review. Crit Rev Environ Sci Technol 51(17):1970–2014
He Y, Pei M, Xue N, Wang L, Guo W (2016) Synthesis of sodium polyacrylate-bentonite using in situ polymerization for Pb2+removal from aqueous solutions. RSC Adv 6:48145–48154
Güçlü G, Al E, Emik S, Iyim TB, Ozgümüs S, Ozyürek M (2010) Removal of Cu2+ and Pb2+ ions from aqueous solutions by starch-graft-acrylic acid/montmorillonite superabsorbent nanocomposite hydrogels. Polym Bull 65:333–346
Atia AA, Doniaa AM, Hussin RA, Rashad RT (2009) Swelling and metal ion uptake characteristics of kaolinite containing poly [(acrylic acid)-co-acrylamide] hydrogels. Desalin Water Treat 3:73–82
Bhatia M, Rajulapati SB, Sonawane S, Girdhar A (2017) Synthesis and implication of novel poly(acrylic acid)/nanosorbent embedded hydrogel composite for lead ion removal. Sci Rep 7:16413
Zhang M, Wan Y, Wen Y, Li C, Kanwal A (2020) A novel Poly (vinyl alcohol)/carboxymethyl cellulose/yeast double degradable hydrogel with yeast foaming and double degradable property. Ecotoxicol Environ Saf 187:109765
Sarmah D, Karak N (2020) Double network hydrophobic starch based amphoteric hydrogel as an effective adsorbent for both cationic and anionic dyes. Carbohyd Polym 242:116320
Ren J, Wang X, Zhao L, Li M, Yang W (2021) Effective removal of dyes from aqueous solutions by a gelatin hydrogel. J Polym Environ 29(11):3497–3508
El Kony A, Ibrahim AG, Abu El-Farah MH, Abd-Elhai F (2020) Synthesis and characterization of (AAm-co-AHPS)/MMT hydrogel composites for the efficient capture of methylene blue from aqueous solution. Al-Azhar Bull Sci 31(2-A):31–46
Ilgin P, Ozay H, Ozay O (2019) Selective adsorption of cationic dyes from colored noxious effluent using a novel N-tert-butylmaleamic acid based hydrogels. React Funct Polym 142:189–198
Pirbazari E, Saberikhah E, Kozani H (2014) Fe3O4–wheat straw: preparation, characterization and its application for methylene blue adsorption. Water Resources Industry 7:23–37
Hayatolgheibi SH, Ameli F, Moghbeli MR (2022) A simulation study on hydrogel performance for enhanced oil recovery using phase-field method. Sci Rep 12(1):1–19
Tačić A, Ilić-Stojanović S, Nikolić V, Nikolić L, Zdravković A, Najman S, Stojanović S (2016) The synthesis and characterization of poly(N-isopropylacrylamide) hydrogels and residual reactants analysis. In: Book of abstracts of the XXIV Congress of chemists and technologists of Macedonia, Ohrid, Macedonia, 11–14 Sept 2016, p 288.
Wang W, Deng L, Huang P, Xu S, Li X, Lv N et al (2014) Toxicity and in vivo biological effect of the nanoparticular self-supported hydrogel of a thermosensitive copolymer for non-invasive drug delivery. J Biomed Mater Res A 102(1):17–29
El Mansoup A, El Sayed MM, El Nashar RM, Fahmy HM, Abulnour AM (2022) Chemically/electrically-assisted regeneration of polyacrylonitrile-based hydrogel adsorbed heavy metals. Egypt J Chem 65(1):1–2
Mohammadi Z, Shangbin S, Berkland C, Liang JT (2017) Chelator-mimetic multi-functionalized hydrogel: highly efficient and reusable sorbent for Cd, Pb, and As removal from waste water. Chem Eng J 2017(307):496–502
Pourjavadi A, Tehrani ZM, Salimi H, Banazadeh A, Abedini N (2015) Hydrogel nanocomposite based on chitosan-g-acrylic acid and modified nanosilica with high adsorption capacity for heavy metal ion removal. Iran Polym J 24:725–734
Hafez HS, Ali A, Abdel-Mottaleb MSA (2005) Photocatalytic efficiency of titanium dioxide immobilized on PVP/AA hydrogel membranes: a comparative study for safe disposal of wastewater of Remazol Red RB-133 textile dye. Int J Photoenergy 7(4):181–185
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MME: the only author for this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J Polym Environ 31, 2855–2879 (2023). https://doi.org/10.1007/s10924-023-02796-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02796-z