Abstract
Conventional plastics can be substituted for protein-based bioplastics due to their natural origin and their biodegradability. Nevertheless, their properties are inferior to those obtained for synthetic plastics. The chemical crosslinking of these bioplastics with aldehydes could improve their properties to compete in the actual market. Thus, the main goal of this article was to assess the influence of the incorporation of aldehydes with different aliphatic chain length on the physicochemical (crosslinking degree, colour and transparency), mechanical (flexural and tensile behaviour) and functional (water uptake capacity and biodegradability) properties of protein-based bioplastics. In this sense, pea protein, a by-product of food industry, was used as raw material, processing it by injection moulding to obtain the bioplastics. Formaldehyde, glyoxal and glutaraldehyde were the aldehydes used as crosslinking agents. The results show the rise of the mechanical properties with the incorporation of the aldehydes, depending on the degree of crosslinking they generate. All this also causes a consequent loss of the water uptake capacity and an increase in biodegradability time. In conclusion, this work opens a new alternative to develop sustainable bioplastics that can be used in the market.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The usual and excessive use of plastics derived from oil results in an increased risk for the planet. As is well known, plastics generally take hundreds of years to degrade, having a great impact on ecosystems and their biodiversity [1]. Oceans and rivers are affected by them and it is expected that, by 2050, there would be more tons of plastic than fish in ocean [2].
For this reason, there is a preveailing need to look for alternatives to plastic as soon as possible on a day-to-day basis. One of the solutions to this problem is the development of bioplastics, which currently only account for 1% of all plastics used worldwide [3]. However, the definition of bioplastics is controversial, as it incorporates both plastics that: (1) come from biomass (even if they are not biodegradable), such as bio-PET or bio-PE, (2) are biodegradable (even if they come from fossil resources), such as PBAT or PCL, or (3) meet both properties, such as PLA, PHA or proteins blends [4]. In this last category, those bioplastics processed directly from biowastes or by-products, with low added value, play a key role [5] since they reduce dependence on fossil resources and are fully biodegradable [6]. The importance of finding an alternative to traditional plastics is also related to the reduction in the use of limited fossil resources and the consequent emissions of CO2 into the atmosphere to mitigate the greenhouse effect [7]. This fact makes the development of bioplastics from biowaste or by-products of the agri-food industry not a challenge but a necessity in a circular economy context. Therefore, the use of biowaste and by-products with low added value contributes to a more sustainable and circular management of natural resources with the development of the bioeconomy [8,9,10] in line with international policies, such as the European Green Deal and with some of the 2030 Sustainable Development Goals (SDG). Technological research must be aimed at the development of bioplastics with versatile properties that can compete with conventional plastics in the myriad of applications in which they are used today [11].
Several studies have analysed the use of various proteins and polysaccharides for the manufacture of biopolymer-based materials [12]. Among them, soy protein [13,14,15], wheat protein [16, 17] or pea protein [18, 19] stand out. Nevertheless, the properties of protein or polysaccharide-based bioplastics are inferior to those of synthetic plastics [20]. Therefore, crosslinking is a suistable strategy to improve the properties of these bioplastics. The crosslinking can be achieved by a physical or chemical process [21]. Chemical processes generate new and, generally, irreversible covalent bonds [22]. As an example, the use of aldehydes is highly recommended, as they enable the formation of covalent bonds between polymer chains, leading to a microstructural modification of the system [23]. Formaldehyde and glutaraldehyde have been largely studied as crosslinkers, but their toxicity limits their use since aldehydes are toxic when released even at concentrations as low as 3.0 ppm. Despite this, glutaraldehyde is one of the most widely used crosslinking agent for several applications [16, 24, 25]. In this sense, the greatest novelty of this study consists in the analysis of the influence of the length of the aliphatic chain on the crosslinking effect of various aldehydes in protein bioplastics.
Thus, the aim of this work was to develop bioplastics using pea protein (a by-product obtained from the pea pod as raw material, and glycerol, as plasticiser. To this initial formulation, different aldehyde derivatives were added as crosslinking agents (formaldehyde, glyoxal and glutaraldehyde) to modify its microstructure and consequently its physicochemical (degree of crosslinking, colour and transparency), mechanical (through tensile and bending tests) and functional properties (water absorption capacity and soluble matter loss).
Experimental
Materials
Pea protein (protein isolate, ca. 90 wt% protein content) was selected as raw material. It was supplied by Roquette (France), which stabilizes by drying and grinding the peas discarded for quality (due to their small size or presence of visual defects) in the peeling of the pea pods. Therefore, this by-product was obtained as a homogeneous and stable flour. Formaldehyde (1 C atom, 30 g/mol), glyoxal (2 C atoms, 58 g/mol) and glutaraldehyde (5 C atoms, 100 g/mol) were used as crosslinking agents. In addition, glycerol was used as plasticiser. These reagents were supplied by Sigma Aldrich (Germany).
Fabrication of Bioplastic-Material
Sample Preparation
Bioplastic materials from pea protein were obtained by a two-step process. Thus, the raw materials (1.5/1 protein/plasticiser ratio and 1 or 3 wt% of crosslinking agent) were firstly homogenised in a HaakePolylab QC mixer (ThermoHaake, Germany) at 50 rpm for 10 min to produce a blend.
The blends were subsequently subjected to an injection moulding in a MiniJet Piston Injection Moulding System (ThermoHaake) with the following injection parameters: 50 and 130 °C (cylinder and mould temperatures, respectively), 500 bar for 20 s and 200 bar for 200 s (injection and post-injection conditions, respectively). Two different moulds were used: (1) rectangular-shaped specimen (60 × 10 × 1-mm) and (2) dumbell-shaped specimen (type V) according to ISO 527-1:2012 [26].
Crosslinking
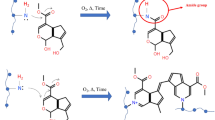
Different aldehydes were used as crosslinking agents to carry out a chemical crosslinking on pea protein-based bioplastics (Fig. 1). Specifically, three different aldehydes varying the aliphatic chain were used: formaldehyde (1 and 3%), glyoxal (3%) and glutaraldehyde (3%). These crosslinking agents present a carbon–oxygen double-bond (C=O) whose polarity makes the carbon atom reactive to primary amines, giving rise to chemically crosslinked structures [27]. In this sense, pea protein presented a high concentration of Arginine and Lysine, among others, according to its amino acid profile, which means that the crosslinking with aldehydes may be effective [28]. Formaldehyde generates a NH–C bond, while glutaraldehyde and glyoxal form N=C bonds when they react with biopolymers, resulting in a more tightly crosslinked network in the bioplastics.
Characterization of Bioplastics
Crosslinking Degree
The analysis of the crosslinking degree was carried out using the protocol described by Zárate-Ramírez et al. [16]. Briefly, a portion of each bioplastic (15 × 10 × 1-mm) was immersed in 10 mL of denaturing solution. This solution allows the solubility of the chemically non-crosslinked protein chains. Finally, the protein content of each solution was evaluated using a modification of the Lowry’s method [29]. Thus, the crosslinking degree was obtained with respect to a reference system (0% crosslinked), comparing the percentage of protein chains chemically crosslinked by the different aldehydes.
Colour and Transparency Measurements
The colour was analysed with a KONICA-MINOLTA CM-700D (Japan) spectrocolorimeter. The value of a and b as the CIELAB chromatic coordinates and L′ as the perceptual lightness of each system was obtained. Δa, Δb and ΔL were used to compare the different systems with the reference one.
On the other hand, transparency analysis were carried out by subjecting a rectangular bioplastic (1 mm thick) at a 600 nm of wavelength (spectrophotometer Genesys-20, Thermo Scientific, USA) [19]. Thus, the transmittance (%) of each sample was measured, allowing to calculate their transmittance index (TI, Eq. 1). It is worth mentioning that the reference bioplastic was the one without crosslinking.
Mechanical Properties
Dynamic mechanical analysis (DMA) flexural tests were performed using rectangular probes on a RSA3 dynamic-mechanical analyser (TA Instruments, USA) with a dual cantilever bending geometry. Firstly, strain sweep tests (0.002–1% strain at a constant frequency of 1 Hz) were made to evaluate the linear viscoelastic range of the different systems. Then, frequency sweep tests (0.02–20 Hz) were performed at a constant strain within the linear viscoelastic range obtained from the previous strain sweep tests.
Tensile tests were performed by subjecting the bioplastics (Type V geometry) to an increasing axial force (rate: 10 mm/min) using a Insight 10 kN Electromechanical Testing System (MTS, USA). Different mechanical parameters (Young’s modulus, maximum stress and strain at break) were calculated from stress–strain curves.
All measurements were carried out at room temperature.
Water Uptake Capacity (WUC) and Soluble Matter Loss (SML)
The ASTM D570-98 standard [30] was followed to carried out water uptake capacity measurements. In this way, rectangular samples were previously conditioned in oven at 50 °C (1 h) [Initial Dry Weight]. Then the bioplastic was submerged in distilled water for 24 h, after which they were weighed again [Wet Weight]. Finally, they were dried again at 100 °C for 24 h [Final Dry Weight]. Water Uptake Capacity (WUC) and Soluble Matter Loss (SML) were then determined though these weights using Eqs. (2) and (3):
Water Contact Angle (WCA)
Water contact angle was measured using the sessile drop (5 µL) analysis with an Optical Tensiometer (Theta Flex, Biolin Scientific). WCA values were obtained as the average left and right sides of the deionized water droplets were measured and the average value was calculated.
Biodegradability
The biodegradability of the systems was measured by burying the bioplastics in a composting medium (2:1 farmland: compost, the same inert/organic materials ratio specified by ISO 20200 [30]). At least three rectangular bioplastics of each system were evaluated by unearthing them to be visually evaluated at different days; pictures of all systems were taken to this end. Test was considered finished when the bioplastic could not be unearthed (total biodegradation time).
Statistical Analysis
The results were presented as mean values and their standard deviation (MV ± SD) of at least three replicates. Statistical analyses were carried out using a 95% of confidence level (p < 0.05) using t-test analysis of the statistical package Excel (Microsoft 365, USA).
Results and Discussion
Physicochemical Properties
According to the results shown in Table 1, firstly, a higher concentration of the crosslinking agent led to an increase in the crosslinking degree of the system comparing the values for formaldehyde at 1 and 3%. The increase observed in the crosslinking degree is due to the presence of more binding sites between the different protein chains. Comparing the different crosslinking agents, it was observed that the highest crosslinking was achieved with the molecule with a single carbon atom (formaldehyde). It favours the formation of both intramolecular and intermolecular bonds, being the smallest aldehyde and susceptible to react with N-terminal amino groups and side chains of cysteine, histidine, lysine, tryptophan, and arginine [31]. However, it is demonstrated that an aliphatic chain of two carbon atoms (glyoxal) resulted in a decrease in the degree of crosslinking. It would be because formaldehyde leaves the –NH group within the crosslinked structure while glyoxal and glutaraldehyde do not [32]. The presence of such –NH groups may support the higher crosslinking caused by formaldehyde. On the other hand, when glutaraldehyde was used, a slight increase in the crosslinking of bioplastics is observed when compared to glyoxal, as the reactivity of the molecule is higher [33].The changes derived from the addition of different aldehydes to the initial formulation were also visible on the bioplastics’ final physical aspect.
Firstly, as is shown in Fig. 2, the addition of formaldehyde produced no significant differences in the colour of the bioplastics compared to the reference system. Nevertheless, the use of glutaraldehyde changed the colour of the bioplastic toward a brownish tone, whereas the use of glyoxal produced a complete darkening of the sample. These changes could be produced by Maillard reactions [34, 35], which can also be observed in the colour parameters summarised in Table 2.
Considering the different aldehydes used, the addition of formaldehyde produced bioplastics with no significant differences respect the reference, with high and positive values for both a and b parameters, as proof of the yellow/brownish colour of the bioplastic. Nevertheless, the decrease observed in the colour parameters for the glyoxal system is an indication of the brownish colour observed in Fig. 1. In fact, apart from the colour change, there was a darkening of the bioplastics, as shown by the decrease of the L* parameter (ΔL* lower than 0). Considering the system obtained with glutaraldehyde, there was an increase in the a parameter, as a consequence of the brownish colour of the resulting bioplastic. According to previous studies, the colour changes are consequence of the use of aldehydes for covalent crosslinking of proteins, as derived result of the Maillard reactions [36, 37]. Similar results have been obtained by other authors, since the use of different crosslinking agents produced a variation in the initial colour of the bioplastics, as the darkening of agarose bioplastic films using citric acid [38] or the modification of the colour of gluten-based bioplastics using glutaraldehyde or glyoxal agents [16]. In fact, as commented by Jia et al. [39], the use of aldehydes (cinnamaldehyde) as crosslinking agents produced a darkening and higher opacity of gliadin-based films [40].
On the other hand, the addition of either glyoxal or glutaraldehyde modified the transparency of the systems, producing opaque bioplastics, as shown by the drastic decrease of the transparency index obtained for the bioplastics crosslinked with glyoxal or glutaraldehyde.
Mechanical Properties
The evolution of the crosslinking with different aldehydes was also assessed by measuring the mechanical properties of the resulting bioplastics in both static and dynamic mode.
The results from the dynamic flexural tests are plotted in Fig. 3A. A drastic increase in the E’ values was observed with the addition of all the different aldehydes studied. The profile exhibited is similar to that of the reference system, with an almost constant behaviour over the frequency range studied, although the elastic character of the bioplastics varied with the aliphatic length of the crosslinking agent. E’1 values included in Table 3 were used to study possible significant differences between the systems. In general, the addition of any crosslinking agent produced a reinforcement of the structure, since the elastic moduli of all the systems were higher than that of the reference system.
A correlation between the flexural properties and the number of carbons of the crosslinking agent used was observed (Fig. 4A). Thus, the increase in the aliphatic chain length of the crosslinking agent reduced the E’ values. This correlation revealed that the aliphatic chain length had a greater impact on the elastic modulus (E’) than on the viscous modulus (E’’) since the decreasing slope is sharper. This decrease is greater in the change from formaldehyde to glyoxal or glutaraldehyde, possibly due to the bond change discussed above (–NH–C vs. N=C for formaldehyde and glyoxal or glutaraldehyde, respectively).
Tensile tests were also performed for the bioplastics crosslinking with different aldehydes. In the profiles shown in Fig. 3B, three well-differentiated regions can be observed in all the systems. The first region, linear and with a steep slope, indicates the range of strain in which the material has an elastic behaviour. The second region, also linear but with a lower slope compared to the previous one, indicates the range of strain in which the material has a plastic behaviour. Finally, a third region corresponds to an abrupt drop in stress, produced by the break of the material. Tensile parameters obtained from the different profiles in Fig. 3B are shown in Table 3. The bioplastic obtained with glyoxal (3%) is the system with the highest value of the maximum stress, but with a strain at break lower than that of the other systems, except for the glutaraldehyde one. This fact, together with the high value of Young's modulus, indicates that the use of glutaraldehyde produced less deformable systems, as demonstrated by Pavoni et al. [41] by using glutaraldehyde crosslinking on chitosan films [39]. It could be noted that, in general, the behavior in traction is clearly different with respect to bending. In this sense, the glutaraldehyde system also stood out, allowing little strain before breaking and the lowest stress value. On the other hand, the longer the aliphatic chain, the lower the strain at break (from 0.48 to 0.05 mm/mm), being also lower than the reference system (0.72 mm/mm). Other authors have obtained similar results when applying a heat treatment as an additional crosslinking stage to the fabrication of protein-based bioplastics, with a marked decrease in the strain at break with respect to the reference system [41]. In summary, the correlation between the tensile properties and the number of carbons of the crosslinking agent used (Fig. 4B) showed that the use of glyoxal (C2) or glutaraldehyde (C5) produced systems with a higher Young’s Modulus and a lower strain at break, although their crosslinking degree is lower than for formaldehyde. However, there are many factors to consider, such as the greater reactive sites incorporated by formaldehyde (a greater number of molecules incorporated in the same weight of reagent) or the different bonds formed with the crosslinking agents, which could explain this greater influence on the mechanical properties of bioplastics.
Functional Properties
In addition to the mechanical properties, functional properties of the crosslinked bioplastics were also evaluated. In this sense, water absorption and biodegradability were measured and compared.
The water uptake capacity of bioplastics is showed in Fig. 5 with and without the addition of aldehydes as crosslinking agents. Regarding water absorption, it is observed that there is a clear difference between the reference system and the crosslinked ones. The system composed solely of pea protein and glycerol absorbed its own weight of water (ca. 105%). On the other hand, with the addition of the different crosslinking agents, the water absorption decreased. A higher concentration of formaldehyde, which means a higher crosslinking degree, increased the elastic modulus thus reducing their ability to swell and, as a consequence, to absorb water. This behavior was also observed by Álvarez-Castillo et al. [42] with plasma protein-based bioplastics [43] or Jiménez-Rosado et al. [18, 43] for soy protein-based bioplastics [42].
On the other hand, the use of different crosslinking agents produced modification in the morphology of the resulting bioplastics due to the differences in their aliphatic chain length. Thus, although a greater degree of crosslinking is not showed, the water uptake capacity is reduced with the increase in the aliphatic chain length. This could be due to possible modifications in terms of hydrophobicity or mechanical response of the systems. However, according to the results shown in Fig. 6, although it is true that there is an increase in the contact angle compared to the reference system, the values obtained are not sufficient to consider hydrophobicity as the main cause of the water uptake values obtained. In this sense, it may be caused by a combination of factors such as the greater rigidity of the bonds that prevent the matrix from swelling or the different molecular percentage of the incorporated crosslinking agent.
On the other hand, the soluble matter loss remained constant at 48%, which means that, apart from glycerol, because of the high solubility of pea protein, a small amount of soluble protein was also lost during immersion [44]. These results have been corroborated by Jia et al. [39], who showed that the addition of cinnamaldehyde as crosslinking agent produced a decrease in the water absorption [40].
The biodegradability of the bioplastics was also measured, Fig. 7. A qualitatively analysis of the degradation of the bioplastics was carried out by taking photos at different days after burying the samples in soil and results were shown in Fig. 7. The reference pea protein bioplastic was completely degraded after 10 days. The addition of different aldehydes produced a delay in the degradation of the samples, being higher with the increase in the aliphatic chain length of the crosslinking agent. In this sense, in terms of biodegradation, there was a 40% increase when formaldehyde was added to the formulation (4 days more than the reference one), a 100% increase when using glyoxal (10 days more than the reference one) and a 400% increase when glutaraldehyde was added (30 days more than the reference one). This increase may be due to two factors: (i) the higher mechanical resistance of the bioplastics observed in the mechanical tests and (ii) the antibacterial activity of these aldehydes that killed the bacteria before they could break down the bioplastics [45, 46].
Conclusions
Pea protein-based bioplastics with improved properties were obtained by including different aldehydes as crosslinking agents to the traditional thermomoulding processing of bioplastics.
Regarding the physicochemical properties, a significant and concentration-dependent increase with the degree of crosslinking was observed respect to the reference bioplastic obtained with pea protein and glycerol. Furthermore, the addition of aldehyde-based crosslinking agents led to browning, decreased transparency, and colour change systems, being more evident for the glyoxal and glutaraldehyde bioplastics.
Crosslinking agents modified the properties of bioplastics, especially improving flexural properties in all cases. Although it is difficult to correlate the aliphatic chain with the mechanical properties of bioplastics, it has been found that aldehydes with shorter chains significantly improved the stiffness of bioplastics.
On the other hand, the addition of aldehydes to the initial formulation caused a decrease in the water absorption capacity, although without influencing the soluble matter loss of the resulting bioplastics, which mainly corresponds to the amount of glycerol used as plasticizer in processing. Furthermore, the chemical crosslinking of protein bioplastics delayed their biodegradation time, increasing as the length of the aliphatic chain of the added crosslinking agent increased due to the change in the crosslinking bonds.
In conclusion, the use of aldehydes as crosslinking agents makes the bioplastics more resistance against mechanical stress, losing deformation capacity, as well as a lower water absorption capacity, which is a useful property for applications in which the humidity in the product should not be modified. Nevertheless, it is necessary a more in-depth work in order to establish what are the more significant factors in aliphatic compounds that influence the bioplastic properties.
Data Availability
The data that support the findings of this study are not publicly available due to third part privacy.
References
Ahmed T, Shahid M, Azeem F et al (2018) Biodegradation of plastics: current scenario and future prospects for environmental safety. Environ Sci Pollut Res 25:7287–7298. https://doi.org/10.1007/s11356-018-1234-9
Gill M (2014) Bioplastic: a better alternative to plastics. Int J Res Appl Nat Soc Sci 2:115–120
Barbi S, Macavei LI, Caligiani A et al (2021) From food processing leftovers to bioplastic: a design of experiments approach in a circular economy perspective. Waste Biomass Valorization 12:5121–5130. https://doi.org/10.1007/s12649-021-01376-3
Gironi F, Piemonte V (2011) Bioplastics and petroleum-based plastics: strengths and weaknesses. Energy Sources Part A. https://doi.org/10.1080/15567030903436830
George N, Debroy A, Bhat S et al (2021) Biowaste to bioplastics: an ecofriendly approach for a sustainable future. J Appl Biotechnol Rep. https://doi.org/10.30491/JABR.2021.259403.1318
Felix M, Perez-Puyana V, Romero A, Guerrero A (2017) Development of protein-based bioplastics modified with different additives. J Appl Polym Sci 143:45430
Narayan R (2011) Carbon footprint of bioplastics using biocarbon content analysis and life-cycle assessment. MRS Bull 36:716–721. https://doi.org/10.1557/mrs.2011.210
Confente I, Scarpi D, Russo I (2020) Marketing a new generation of bio-plastics products for a circular economy: the role of green self-identity, self-congruity, and perceived value. J Bus Res 112:431–439. https://doi.org/10.1016/j.jbusres.2019.10.030
Vea EB, Romeo D, Thomsen M (2018) Biowaste valorisation in a future circular bioeconomy. Procedia CIRP 69:591–596. https://doi.org/10.1016/j.procir.2017.11.062
Lamberti FM, Román-Ramírez LA, Wood J (2020) Recycling of bioplastics: routes and benefits. J Polym Environ 28:2551–2571. https://doi.org/10.1007/s10924-020-01795-8
Degli Esposti M, Morselli D, Fava F et al (2021) The role of biotechnology in the transition from plastics to bioplastics: an opportunity to reconnect global growth with sustainability. FEBS Open Biol 11:967–983. https://doi.org/10.1002/2211-5463.13119
Omrani-Fard H, Abbaspour-Fard MH, Khojastehpour M, Dashti A (2020) Gelatin/whey protein- potato flour bioplastics: fabrication and evaluation. J Polym Environ 28:2029–2038. https://doi.org/10.1007/s10924-020-01748-1
Felix M, Martínez I, Aguilar JM, Guerrero A (2018) Development of Biocomposite Superabsorbent Nanomaterials: effect of processing technique. J Polym Environ 26:4013–4018. https://doi.org/10.1007/s10924-018-1262-z
Fernández-Espada L, Bengoechea C, Sandía JA et al (2019) Development of novel soy-protein-based superabsorbent matrixes through the addition of salts. J Appl Polym Sci 136:47012. https://doi.org/10.1002/app.47012
Gamero S, Jiménez-Rosado M, Romero A et al (2019) Reinforcement of soy protein-based bioplastics through addition of lignocellulose and injection molding processing conditions. J Polym Environ 27:1285–1293. https://doi.org/10.1007/s10924-019-01430-1
Zárate-Ramírez LS, Romero A, Martínez I et al (2014) Effect of aldehydes on thermomechanical properties of gluten-based bioplastics. Food Bioprod Process 92:20–29. https://doi.org/10.1016/j.fbp.2013.07.007
Gómez-Heincke D, Martínez I, Partal P et al (2016) Development of antimicrobial active packaging materials based on gluten proteins. J Sci Food Agric 96:3432–3438. https://doi.org/10.1002/jsfa.7525
Jiménez-Rosado M, Bouroudian E, Perez-Puyana V et al (2020) Evaluation of different strengthening methods in the mechanical and functional properties of soy protein-based bioplastics. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.121517
Perez-Puyana V, Felix M, Romero A, Guerrero A (2016) Effect of the injection moulding processing conditions on the development of pea protein-based bioplastics. J Appl Polym 133:1–9. https://doi.org/10.1002/app.43306
Felix M, Perez-Puyana V, Romero A, Guerrero A (2017) Production and characterization of bioplastics obtained by injection moulding of various protein systems. J Polym Environ 25:91–100. https://doi.org/10.1007/s10924-016-0790-7
Hassan MM, Fowler IJ (2022) Thermal, mechanical, and rheological properties of micro-fibrillated cellulose-reinforced starch foams crosslinked with polysiloxane-based cross-linking agents. Int J Biol Macromol 205:55–65. https://doi.org/10.1016/j.ijbiomac.2022.02.017
Azeredo HMC, Waldron KW (2016) Crosslinking in polysaccharide and protein films and coatings for food contact – A review. Trends Food Sci Technol 52:109–122. https://doi.org/10.1016/j.tifs.2016.04.008
Silva CJSM, Sousa F, Gübitz G, Cavaco-Paulo A (2004) Chemical modifications on proteins using glutaraldehyde. Food Technol Biotechnol 42:51–56
Gomes SR, Rodrigues G, Martins GG et al (2013) In vitro evaluation of crosslinked electrospun fish gelatin scaffolds. Mater Sci Eng C-Materials Biol Appl 33:1219–1227. https://doi.org/10.1016/j.msec.2012.12.014
Zhang X, Tang K, Zheng X (2016) Electrospinning and crosslinking of COL/PVA nanofiber-microsphere containing salicylic acid for drug delivery. J Bionic Eng 13:143–149. https://doi.org/10.1016/S1672-6529(14)60168-2
UNE-EN ISO 527–1 2012. Plastics Determination of tensile properties Part 1: General principles ISO Geneva Switzerland
Feeney RE, Blankenhorn G, Dixon HBF (1975) Carbonyl-amine reactions in protein chemistry. Advances in Protein Chemistry. Elsevier, Cola de Ratón, pp 135–203
Audic J-L, Chaufer B (2005) Influence of plasticizers and crosslinking on the properties of biodegradable films made from sodium caseinate. Eur Polym J 41:1934–1942. https://doi.org/10.1016/j.eurpolymj.2005.02.023
Markwell MAK, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87:206–210. https://doi.org/10.1016/0003-2697(78)90586-9
ASTM (American Society for Testing and Materials) (2005) Standard test method for plastic. Annual book of ASTM standards. American Society for Testing and Materials, Philadelphia PA
ISO 20200:2004 (2004) Plastics: determination of the degree of disintegration of plastic materials under simulated composting conditions in a laboratory-scale test. ISO, Geneva
Metz B, Kersten GFA, Hoogerhout P et al (2004) Identification of formaldehyde-induced modifications in proteins. J Biol Chem 279:6235–6243. https://doi.org/10.1074/jbc.M310752200
Hoffman EA, Frey BL, Smith LM, Auble DT (2015) Formaldehyde crosslinking: a tool for the study of chromatin complexes. J Biol Chem 290:26404–26411. https://doi.org/10.1074/jbc.R115.651679
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–802. https://doi.org/10.2144/04375RV01
Gerrard J, Brown P, Fayle S (2002) Maillard crosslinking of food proteins I: the reaction of glutaraldehyde, formaldehyde and glyceraldehyde with ribonuclease. Food Chem 79:343–349. https://doi.org/10.1016/S0308-8146(02)00174-7
Montha S, Suwandittakul P, Poonsrisawat A et al (2016) Maillard reaction in natural rubber latex: characterization and physical properties of solid natural rubber. Adv Mater Sci Eng 2016:1–6. https://doi.org/10.1155/2016/7807524
Amaya-Farfan J, Rodriguez-Amaya DB (2021) The Maillard reactions. Chemical changes during processing and storage of foods. Elsevier, Amsterdam, pp 215–263
Gan C-Y, Cheng L-H, Easa AM (2009) Assessment of cross-linking in combined cross-linked soy protein isolate gels by microbial transglutaminase and maillard reaction. J Food Sci 74:C141–C146. https://doi.org/10.1111/j.1750-3841.2009.01053.x
Jia F, Wang JJ, Huang Y et al (2021) Development and characterization of gliadin-based bioplastic films enforced by cinnamaldehyde. J Cereal Sci 99:103208. https://doi.org/10.1016/j.jcs.2021.103208
Awadhiya A, Kumar D, Verma V (2016) Crosslinking of agarose bioplastic using citric acid. Carbohydr Polym 151:60–67. https://doi.org/10.1016/j.carbpol.2016.05.040
Pavoni JMF, dos Santos NZ, May IC et al (2021) Impact of acid type and glutaraldehyde crosslinking in the physicochemical and mechanical properties and biodegradability of chitosan films. Polym Bull 78:981–1000. https://doi.org/10.1007/s00289-020-03140-4
Álvarez-Castillo E, Bengoechea C, Guerrero A (2020) Effect of pH on the properties of porcine plasma-based superabsorbent materials. Polym Test. https://doi.org/10.1016/j.polymertesting.2020.106453
Jiménez-Rosado M, Rubio-Valle JF, Perez-Puyana V et al (2020) Use of heat treatment for the development of protein-based bioplastics. Sustain Chem Pharm. https://doi.org/10.1016/j.scp.2020.100341
Jiménez-Rosado M, Rubio-Valle JF, Perez-Puyana V et al (2021) Comparison between pea and soy protein-based bioplastics obtained by injection molding. J Appl Polym Sci 138:50412. https://doi.org/10.1002/app.50412
Perez V, Felix M, Romero A, Guerrero A (2016) Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod Process 97:100–108. https://doi.org/10.1016/j.fbp.2015.12.004
R. R, Madhavan A, Philip E, et al (2021) Sugarcane bagasse derived nanocellulose reinforced with frankincense (Boswellia serrata): physicochemical properties, biodegradability and antimicrobial effect for controlling microbial growth for food packaging application. Environ Technol Innov 21:101335. https://doi.org/10.1016/j.eti.2020.101335
Acknowledgements
This study was financially supported by MCIN/AEI/10.13039/501100011033/FEDER, UE, through the project PID2021-124294OB-C21. Authors acknowledge their support. Authors also acknowledge for the predoctoral grant of Mercedes Jiménez Rosado (FPU17/01718) and the postdoctoral contract of Víctor Manuel Pérez-Puyana (“Contratación de Personal Investigador Doctor”) supported by the European Social Fund and Junta de Andalucía (PAIDI DOCTOR – Convocatoria 2019/2020, DOC_00586).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Ministerio de Ciencia e Innovación-Agencia Estatal de Investigación (MCI/AEI/FEDER, EU) from the Spanish Government (Ref. PID2021-124294OB-C21). Junta de Andalucía and European Social Fund (Ref. PAIDI DOCTOR – Convocatoria 2019/2020, DOC_00586). Ministerio de Educación y Formación Profesional (Ref. FPU17/01718).
Author information
Authors and Affiliations
Contributions
Conceptualization: VPP and AR; methodology, DE and MJR; investigation, DE. and VPP; resources, AR; data curation, MJR; writing—original draft preparation, VPP and MJR; writing—review and editing, AR and IM; visualization, AR and IM; supervision, AR and IM; funding acquisition, AR.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perez-Puyana, V., Jiménez-Rosado, M., Escribano, D. et al. Influence of the Aliphatic Chain Length on the Crosslinking Properties of Aldehydes on Sustainable Bioplastics Obtained from Pea Protein. J Polym Environ 30, 5163–5172 (2022). https://doi.org/10.1007/s10924-022-02571-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02571-6