Abstract
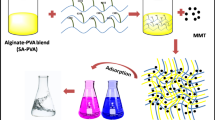
Semi-interpenetrating networks (semi-IPNs) of Xanthan gum/poly (acrylic acid) containing Cloisite 15A were prepared via radical polymerization using a novel acrylic-urethane crosslinker. Response surface methodology (RSM) was used to optimize the conversion, swelling, and networking of synthesized hydrogels as a function of three variables: xanthan gum, cross-linker, and clay. FT-IR, UV–Vis, XRD, BET, TEM and SEM techniques were used to analyze and confirm the formation and efficiency of the synthesized hydrogels. The surface areas of the XG/PAA and XG/PAA/Cloisite 15A samples were 1.3536 and 1.759 m2 g−1, respectively, demonstrating that Cloisite 15A nano-sheets are effective in increasing hydrogel surface area. Adsorption behavior of Co2+, Cu2+, Ni2+ by optimized hydrogel was defined using kinetic and isotherm models. The kinetic analysis revealed that the pseudo-second-order best explains the kinetic mechanism (R2 = 0.999). At 15,000 g mL−1 initial concentration, the adsorption capacity for Co2+, Cu2+, Ni2+ were 436.62, 530.14, and 511.74 mg g−1, respectively. The results show that the Xanthan-based hydrogel has a porous structure and high adsorption capacity. Moreover, the cycling absorption performance of XG/PAA/Cloisite 15A is promising. Metal ions were removed by XG/PAA/Cloisite 15A at about 45 and 30% in the 1st and 5th cycles, respectively.
Graphical Abstract








Similar content being viewed by others
References
Emiliani J, Oyarce WGL, Bergara CD, Salvatierra LM, Novo LAB, Pérez LM (2020) Variations in the phytoremediation efficiency of metal-pollutedwater with salvinia biloba: Prospects and toxicological impacts. Water (Switzerland). https://doi.org/10.3390/W12061737
Tang S, Lin L, Wang X, Yu A, Sun X (2021) Interfacial interactions between collected nylon microplastics and three divalent metal ions (Cu(II), Ni(II), Zn(II)) in aqueous solutions. J Hazard Mater 403(2020):123548. https://doi.org/10.1016/j.jhazmat.2020.123548
He X et al (2021) Quantum dots encoded white-emitting polymeric superparticles for simultaneous detection of multiple heavy metal ions. J Hazard Mater 405:124263. https://doi.org/10.1016/j.jhazmat.2020.124263
Garcia P et al (2020) Inexpensive organic materials and their applications towards heavy metal attenuation in water from Southern Peru. Water (Basel). https://doi.org/10.3390/w12102948
Shabtai IA, Lynch LM, Mishael YG (2021) Designing clay-polymer nanocomposite sorbents for water treatment: A review and meta-analysis of the past decade. Water Res 188:116571. https://doi.org/10.1016/j.watres.2020.116571
Jaspal D, Malviya A (2020) Composites for wastewater purification: a review. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.125788
Sharifpour E, Alipanahpour Dil E, Asfaram A, Ghaedi M, Goudarzi A (2019) Optimizing adsorptive removal of malachite green and methyl orange dyes from simulated wastewater by Mn-doped CuO-nanoparticles loaded on activated carbon using CCD-RSM: mechanism, regeneration, isotherm, kinetic, and thermodynamic studies. Appl Organomet Chem 33(3):1–14. https://doi.org/10.1002/aoc.4768
Olad A, Doustdar F, Gharekhani H (2020) Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf, A 601:124962. https://doi.org/10.1016/j.colsurfa.2020.124962
Pandey S, Do JY, Kim J, Kang M (2020) Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int J Biol Macromol 143(2):60–75. https://doi.org/10.1016/j.ijbiomac.2019.12.002
Wahlström N, Steinhagen S, Toth G, Pavia H, Edlund U (2020) Ulvan dialdehyde-gelatin hydrogels for removal of heavy metals and methylene blue from aqueous solution. Carbohyd Polym 249:116841. https://doi.org/10.1016/j.carbpol.2020.116841
Mignon A, de Belie N, Dubruel P, van Vlierberghe S (2019) Superabsorbent polymers: a review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’ derivatives. Eur Polymer J 117:165–178. https://doi.org/10.1016/j.eurpolymj.2019.04.054
Sivakumar R, Lee NY (2022) Adsorptive removal of organic pollutant methylene blue using polysaccharide-based composite hydrogels. Chemosphere 286(P3):131890. https://doi.org/10.1016/j.chemosphere.2021.131890
Khademian E, Salehi E, Sanaeepur H, Galiano F, Figoli A (2020) A systematic review on carbohydrate biopolymers for adsorptive remediation of copper ions from aqueous environments-part A: classification and modification strategies. Sci Total Environ 738:139829. https://doi.org/10.1016/j.scitotenv.2020.139829
Riaz T, Iqbal MW, Jiang B, Chen J (2021) A review of the enzymatic, physical, and chemical modification techniques of xanthan gum. Int J Biol Macromol 186:472–489. https://doi.org/10.1016/j.ijbiomac.2021.06.196
Ahmad S, Ahmad M, Manzoor K, Purwar R, Ikram S (2019) A review on latest innovations in natural gums based hydrogels: preparations & applications. Int J Biol Macromol 136:870–890. https://doi.org/10.1016/J.IJBIOMAC.2019.06.113
Abu Elella MH, Goda ES, Abdallah HM, Shalan AE, Gamal H, Yoon KR (2021) Innovative bactericidal adsorbents containing modified xanthan gum/montmorillonite nanocomposites for wastewater treatment. Int J Biol Macromol 167:1113–1125. https://doi.org/10.1016/j.ijbiomac.2020.11.065
Abu Elella MH et al (2021) Xanthan gum-derived materials for applications in environment and eco-friendly materials: a review. J Environ Chem Eng 9(1):104702. https://doi.org/10.1016/j.jece.2020.104702
Wang W et al (2020) High-performance two-dimensional montmorillonite supported-poly(acrylamide-co-acrylic acid) hydrogel for dye removal. Environ Pollut 257:113574. https://doi.org/10.1016/j.envpol.2019.113574
Pereira AGB, Rodrigues FHA, Paulino AT, Martins AF, Fajardo AR (2021) Recent advances on composite hydrogels designed for the remediation of dye-contaminated water and wastewater: a review. J Clean Prod 284:124703. https://doi.org/10.1016/j.jclepro.2020.124703
Pooresmaeil M, Namazi H (2019) Application of polysaccharide-based hydrogels for water treatments. Elsevier Inc, Amterdam
Jana S, Ray J, Mondal B, Tripathy T (2019) Efficient and selective removal of cationic organic dyes from their aqueous solutions by a nanocomposite hydrogel, katira gum-cl-poly(acrylic acid-co-N, N-dimethylacrylamide)@bentonite. Appl Clay Sci 173(2018):46–64. https://doi.org/10.1016/j.clay.2019.03.009
Thakur B et al (2020) Designing of bentonite based nanocomposite hydrogel for the adsorptive removal and controlled release of ampicillin. J Mol Liq 319:114166. https://doi.org/10.1016/j.molliq.2020.114166
Zhang T et al (2021) Removal of heavy metals and dyes by clay-based adsorbents: from natural clays to 1D and 2D nano-composites. Chem Eng J 420(P2):127574. https://doi.org/10.1016/j.cej.2020.127574
Ismail N, Nazri NK, Abdullah AA, Wan Nik WMN, Wright LJ (2021) Dataset in characterization of the polymer produced using different method of synthesis polychloromethylstyrene (PCMS) with clay and without clay. Data Brief 34:106738. https://doi.org/10.1016/j.dib.2021.106738
Zhang T et al (2020) Removal of heavy metals and dyes by clay-based adsorbents: from natural clays to 1D and 2D nano-composites. Chem Eng J. https://doi.org/10.1016/j.cej.2020.127574
Lv Q, Hu X, Zhang X, Huang L, Liu Z, Sun G (2019) Highly efficient removal of trace metal ions by using poly(acrylic acid) hydrogel adsorbent. Mater Des 181:107934. https://doi.org/10.1016/j.matdes.2019.107934
Hajikhani M, Moradi M, Shahrousvand M (2020) Effect of cross-Linker types on properties of cross-linked poly (acrylic acid) as the superabsorbent. IChEC, pp. 15–17.
Hajikhani M, Khanghahi MM, Shahrousvand M, Mohammadi-Rovshandeh J, Babaei A, Khademi SMH (2019) Intelligent superabsorbents based on a xanthan gum/poly (acrylic acid) semi-interpenetrating polymer network for application in drug delivery systems. Int J Biol Macromol 139:509–520. https://doi.org/10.1016/j.ijbiomac.2019.07.221
Behroozi M, Pakizeh M (2017) Study the effects of Cloisite15A nanoclay incorporation on the morphology and gas permeation properties of Pebax2533 polymer. J Appl Polym Sci 134(37):1–11. https://doi.org/10.1002/app.45302
Olad A, Pourkhiyabi M, Gharekhani H, Doustdar F (2018) Semi-IPN superabsorbent nanocomposite based on sodium alginate and montmorillonite: reaction parameters and swelling characteristics. Carbohyd Polym 190:295–306. https://doi.org/10.1016/j.carbpol.2018.02.088
Mittal H, Kumar V et al (2016) Adsorption of methyl violet from aqueous solution using gum xanthan/Fe3O4 based nanocomposite hydrogel. Int J Biol Macromol 89:1–11. https://doi.org/10.1016/j.ijbiomac.2016.04.050
Makhado E, Pandey S, Nomngongo PN, Ramontja J (2018) Preparation and characterization of xanthan gum-cl-poly(acrylic acid)/o-MWCNTs hydrogel nanocomposite as highly effective re-usable adsorbent for removal of methylene blue from aqueous solutions. J Colloid Interface Sci 513:700–714. https://doi.org/10.1016/j.jcis.2017.11.060
Likhitha M, Sailaja RRN, Priyambika VS, Ravibabu MV (2014) Microwave assisted synthesis of guar gum grafted sodium acrylate/cloisite superabsorbent nanocomposites: reaction parameters and swelling characteristics. Int J Biol Macromol 65:500–508. https://doi.org/10.1016/j.ijbiomac.2014.02.008
Peighambardoust SJ, Foroutan R, Peighambardoust SH, Khatooni H, Ramavandi B (2021) Decoration of Citrus limon wood carbon with Fe3O4 to enhanced Cd2+ removal: a reclaimable and magnetic nanocomposite. Chemosphere 282:131088. https://doi.org/10.1016/J.CHEMOSPHERE.2021.131088
Kumar Sharma A et al (2019) Efficient capture of eosin yellow and crystal violet with high performance xanthan-acacia hybrid super-adsorbent optimized using response surface methodology. Colloids Surf B 175:314–323. https://doi.org/10.1016/J.COLSURFB.2018.12.017
Zeng L et al (2021) Synthesis of cross-linked carboxyl modified polyvinyl alcohol and its application in selective adsorption separation of Cu(II) from Cd(II) and Ni(II). J Polym Environ 29(1):28–37. https://doi.org/10.1007/s10924-020-01847-z
Hosseini H et al (2022) A novel environmentally friendly nanocomposite aerogel based on the semi-interpenetrating network of polyacrylic acid into Xanthan gum containing hydroxyapatite for efficient removal of methylene blue from wastewater. Int J Biol Macromol 201:133–142. https://doi.org/10.1016/J.IJBIOMAC.2021.12.166
Tang L et al (2021) An efficient chitosan-based adsorption material containing phosphoric acid and amidoxime groups for the enrichment of Cu(II) and Ni(II) from water. J Mol Liq 331:115815. https://doi.org/10.1016/J.MOLLIQ.2021.115815
Boughrara L et al (2021) Removal of Zn(II) and Ni(II) heavy metal ions by new alginic acid-ester derivatives materials. Carbohyd Polym 272:118439. https://doi.org/10.1016/J.CARBPOL.2021.118439
Jana S, Ray J, Mondal B, Pradhan SS, Tripathy T (2018) pH responsive adsorption/desorption studies of organic dyes from their aqueous solutions by katira gum-cl-poly(acrylic acid-co-N-vinyl imidazole) hydrogel. Colloids Surf A 553:472–486. https://doi.org/10.1016/J.COLSURFA.2018.06.001
Mohammadzadeh Pakdel P, Peighambardoust SJ, Arsalani N, Aghdasinia H (2022) Safranin-O cationic dye removal from wastewater using carboxymethyl cellulose-grafted-poly(acrylic acid-co-itaconic acid) nanocomposite hydrogel. Environ Res 212:113201. https://doi.org/10.1016/J.ENVRES.2022.113201
Jana S, Ray J, Mondal B, Tripathy T (2019) Efficient and selective removal of cationic organic dyes from their aqueous solutions by a nanocomposite hydrogel, katira gum-cl-poly(acrylic acid-co-N, N-dimethylacrylamide)@bentonite. Appl Clay Sci 173:46–64. https://doi.org/10.1016/J.CLAY.2019.03.009
Thakur S et al (2022) Bentonite-based sodium alginate/ dextrin cross-linked poly (acrylic acid) hydrogel nanohybrids for facile removal of paraquat herbicide from aqueous solutions. Chemosphere 291:133002. https://doi.org/10.1016/J.CHEMOSPHERE.2021.133002
Sharma G, Kumar A, Ghfar AA, García-Peñas A, Naushad M, Stadler FJ (2022) Fabrication and characterization of xanthan gum-cl-poly(Acrylamide-co-alginic acid) hydrogel for adsorption of cadmium ions from aqueous medium. Gels. https://doi.org/10.3390/gels8010023
Makhado E, Pandey S, Ramontja J (2018) Microwave assisted synthesis of xanthan gum-cl-poly (acrylic acid) based-reduced graphene oxide hydrogel composite for adsorption of methylene blue and methyl violet from aqueous solution. Int J Biol Macromol 119:255–269. https://doi.org/10.1016/j.ijbiomac.2018.07.104
Gürkan EH, İlyas B, Tibet Y (2021) Adsorption performance of heavy metal ions from aqueous solutions by a waste biomass based hydrogel: comparison of isotherm and kinetic models. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1873314
Peighambardoust SJ, Aghamohammadi-Bavil O, Foroutan R, Arsalani N (2020) Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int J Biol Macromol 159:1122–1131. https://doi.org/10.1016/j.ijbiomac.2020.05.093
Wang L, Shi C, Pan L, Zhang X, Zou JJ (2020) Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: a review. Nanoscale 12(8):4790–4815. https://doi.org/10.1039/c9nr09274a
Abu Elella MH, Sabaa MW, ElHafeez EA, Mohamed RR (2019) Crystal violet dye removal using crosslinked grafted xanthan gum. Int J Biol Macromol 137:1086–1101. https://doi.org/10.1016/j.ijbiomac.2019.06.243
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Esmaeildoost, F., Shahrousvand, M., Goudarzi, A. et al. Optimization of Xanthan Gum/Poly(acrylic acid)/Cloisite 15A Semi-IPN Hydrogels for Heavy Metals Removal. J Polym Environ 30, 4271–4286 (2022). https://doi.org/10.1007/s10924-022-02501-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02501-6