Abstract
PLA grafting on chitosan has been successfully prepared with two different methods: a direct grafting method and the ROP method. The thermal properties showed that the copolymerization of PLA on the chitosan’s chain by direct grafting is more thermostable than the one obtained by the ROP method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
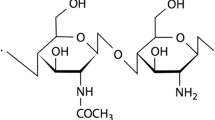
Chitosan is a natural polymer derived from chitin which arouses a lot of interest because of its biodegradability, biocompatibility and its bacteriostatic and fungistatic properties. This material is film-forming and is used to manufacture films without using any additive. More over, the chitosan film has good barrier properties against gas and good mechanical properties (except elasticity). Thanks to these qualities, several studies have been launched on the possible applications of chitosan, to preserve or to extend shelf life from some fresh products, such as fruits and vegetables, meat, and seafood. These studies show that it is possible to use chitosan as an active packaging material. However, there are some limitations. Indeed, it is steam sensitive due to its ability to form a big number of hydrogen bonds (two hydroxyl functions and one amine function in each motif repetition); it is also hard or brittle, and it needs to be associated to another polymer to overcome this kind of problem. That’s why some chemical modifications are interesting techniques to make the chitosan compatible with other polymers. Among the possible solutions, the copolymerization method by grafting may be one of the best methods to combine synthetic polymers with chitosan. In the literature, polymer grafting on the chitosan’s chains has been widely studied, for example the poly (methyl methacrylate) [1], the polyacrylamide [2], the poly (ethylene–glycol) [3], [4], [5], the poly (vinyl alcohol) [6], the polyacrylonitrile [7] and the poly(vinyl acetate) [8]. More over, a pH-fluctuation sensitive hydrogel has been synthesized [9] by direct grafting of d, l-lactic acid on chitosan without using any catalyst. Besides, with the Ring Opening Polymerization method (ROP), l-Lactide has been employed [10] to synthesize chitin-grafted-PLA copolymers.
In this survey, the copolymerization by poly (lactic acid) grafting on chitosan’s chains has been done, with the method described in [9] and [10]; some modifications have been brought in the solvent system and in the catalyst’s use. This work aims at synthesizing and characterizing the chitosan-grafted-PLA copolymers thanks to two different methods. The first method is direct grafting (DG) of d,l-lactic acid on chitosan by using para toluene sulfonic acid as a catalyst. The second method is the Ring Opening Polymerization method (ROP) of l-lactide by using triethylamine (TEA) as an activator (H + selective sensor). In the future, such chitosan-grafted-PLA copolymers could be used directly as a packaging material or as compatibilizing agent in the chitosan/PLA mixtures. The Infrared Heterodyne Spectroscopy (IRTF), the Differential Scanning Calorimetry (DSC) and the solubility test methods have been employed to characterize the grafted copolymers.
Materials and Methods
Materials
The chitosan used was a commercial material obtained from shrimp shell (Les Pêcheries Marinard Ltd., Canada). Its degree of deacetylation was 80–85%, determined by a colloidal method. d, l-Lactide used was a commercial material obtained from Sigma–Aldrich (USA).
Sample Preparation
PLA grafting on chitosan.
Two different methods have been used:
-
The Ring Opening Polymerization method (ROP) of l-lactide, in order to graft PLA on the chitosan’s chain,
-
The direct grafting method (DG) using the d,l-lactic acid monomer.
Figure 1 shows the PLA-grafting process on the chitosan’s chain by the Ring Opening Polymerization method. The chitosan (1.0 g) has been put in 300 ml of 1% acetic acid. We have filtered the solution, put it into a reactor (500 ml) and purged it with nitrogen during a 30 min period. After that, the l-lactide (4.0 g) has been added and the solution containing the reactants has been heated at 80 °C. Then, we added 0.5 ml of triethanolamine to the solution and we have agitated it during 24 h.
The result has been treated in a big quantity of acetone to obtain a precipitate which has been vacuum-dried at ambient temperature. Finally, the homopolymers have been extracted with methanol during 24 h on a Soxhlet extractor.
Chitosan-g-PLA Synthesis by Direct Grafting with the Qu and Al Method [9]
The chitosan-g-PLA synthesis by direct grafting is illustrated in the Fig. 2b. The chitosan (3.0 g) was solubilized in 300 ml of 2% lactic acid. The resulting solution has been put into a reactor (500 ml), agitated with an Ultra-Turrax® homogenizer and purged with nitrogen during 2 h. After that, 9 ml of 85% d,l lactic acid (~ 7 g) have been added, as well as 1 × 10−2 mol of p-Toluene Sulfonic Acid (p-TSA). The reaction occurred at 80 °C and lasted 24 h; then the content of the reactor has been poured into a Petri dish, and dried at 50 °C. The homopolymers -or salts- formed during the grafting reaction have been extracted from the films, obtained by extraction with methanol on a Soxhlet extractor.
Thermal Analysis
The thermal characteristics of the blends were determined by using a Differential Scanning Calorimeter method (DSC) (TA instrument-USA) cooled with a liquid nitrogen circulation. Samples (8–10 mg) were cut from a sample specimen after conditioning and placed in sealed aluminium pans. In DSC analysis, the method [11] has been used with minor modification. For each sample, the following thermal cycle was applied: a first scan was made from 30 °C to 190 °C, then 1 min at 190 °C, and then the sample was cooled rapidly to 30 °C; it has been kept 3 min at 30 °C, and a second scan up to 250 °C was made again. The scanning rate was 10 °C/min, and an empty pan was used as a reference.
IRTF Spectroscopy Analysis
The samples were prepared in 0.50 mm-thick KBr pellets, obtained by mixing 3–5 mg of powder films (extra fine) with 200 mg of dried KBr. The IRTF spectra between 4,000 and 400 cm−1 were recorded using a “Protégé 460. E. S. P. FTIR Spectrometer” device from Nicolet (Madison, WI, USA). All spectra were obtained at ambient temperature with a resolution of 4 cm−1, and 16 scans were carried out for each sample.
Solubility Test and Grafting Efficiency
The grafting efficiency is calculated with the following equation:
where m2, m1 and m3 stand respectively for: m2 = mass of chitosan-g-PLA, m1 = initial mass of chitosan, m3 = mass of the initial monomer. For the solubility tests, 10–20 mg of chitosan-g-PLA are dissolved into different solvents, using a magnetic agitation.
Results and Discussion
Solubility Attempts and Grafting Efficiency
The chitosan’s solubility and the solubility of both chitosan-g-PLA copolymers in different solvents are presented in Table 1. We can see that the solubilities of both chit-g-PLA copolymers differ from the solubility of pure chitosan. The chitosan is soluble in an acidified aqueous solution, whereas none of the two copolymers are soluble, whatever the tested solvents. Distilled water only caused both copolymers to swell. This difference in solubility confirms that a chemical modification occurred on the chitosan’s chain. The grafting efficiencies for both methods are close, around 46% for direct grafting and 47% for the ROP method.
IRTF Spectrometry Characterization
To confirm that PLA grafting on chitosan really occurred, the chitosan IRTF spectra, the l-lactide spectra, the d, l-lactic acid spectra and both chitosan-grafted-PLA spectra have been recorded and compared. Fig. 2 shows all these IRTF spectra: the d, l-lactic acid and the chitosan-grafted-PLA by direct grafting. This figure clearly shows that the chitosan-g-PLA copolymer infrared spectrum has four new peaks at 1,742, 1,453, 1,589 and 1,207 cm−1. The band at 1,453 cm−1 has been linked to the CH3 vibrating deformation. The two peaks at 1,589 and 1,207 cm−1 represent respectively the N–H and C–OH valence vibration. The peak at 1,742 cm−1 represents the C=O (ester group) valence vibration. Compared to the C=O absorption in the lactic acid, (at 1,720 cm−1) the C=O band of the grafted chitosan appears at a higher frequency. Thus, this result confirms the copolymerization by direct grafting of the d, l-lactic acid monomer, on the chitosan’s chain.
The chitosan IRTF spectra, the l-lactide spectra and the chitosan-g-PLA by ROP spectra are represented in Fig. 3. Compared to the chitosan IR-spectrum, the chitosan-g-PLA copolymer spectra shows three new peaks at 1,734, 1,629 and 1,527 cm−1. The amide I peak in the spectrum of pure chitosan moved from 1,652 to 1,629 cm−1 in grafted chitosan. The amines peak at 1,558 cm−1 in the spectrum of pure chitosan moved to 1,527 cm−1 in grafted chitosan. The small peak at 1,734 cm−1showing the esters, as well as the strong peak at 1,761 cm−1 showing the l-lactide carbonyl group, confirm both the grafting by the ROP method.
The chitosan crystallinity index decreases clearly, as shown by the IRTF spectra. This is due to PLA grafting. The crystallinity index values are 0.59 for the chitosan; 0.14 for the chit-g-PLA (DG) and 0.21 for the chit-g-PLA (ROP), see Table 2.
Thermal Properties
Both chitosan-g-PLA copolymers thermal properties are different from those from pure chitosan (Figs. 4 and 5). Figure 3 shows the DSC thermograms for the grafted copolymers and for pure chitosan obtained after the first heating. The two chitosan grafted copolymers don’t show any endothermic peak, while pure chitosan has a high endothermic peak around 91 °C, due to the evaporation of the water present in the chitosan matrices. This result could indicate that grafting decreases the number of free-NH2 and free-OH, which are responsible for the chitosan’s hygroscopy.
During the second DSC sweep (Fig. 5), the thermal degradation of pure chitosan occurs at a highest temperature than the one for grafted chitosan. That could indicate that grafts thermal degradation occurs before the thermal degradation of the main chitosan’s chain. This result goes in the same way that what was showed in [12]: indeed they noticed that the thermal degradation rate related to grafted chitosan copolymer is far higher than the one related to pure chitosan. The thermal degradation temperatures (Tdeg) for chitosan and grafted chitosan (Table 2) are ordered as follow:
Tdeg for chitosan > Tdeg for chit-g-PLA by DG > Tdeg for chit-g-PLA by ROP.
Figure 5 shows that the Glass Transition Temperature Tg for the chitosan is 202 °C, and those for the two copolymers are respectively 144 °C for direct grafting and 149 °C for the ROP method. A lower Tg for grafted chitosan could be due to the suppression of some hydrogen bonds between the chitosan’s chains. In other words, PLA grafting on chitosan increases chitosan’s chain flexibility.
Conclusion
PLA grafting on chitosan has been successfully prepared with two different methods: direct grafting (DG) method and the ROP method.
With the conditions used to conduct this study, the grafting efficiencies for both methods were similar (Table 2). PLA grafting on a chitosan’s chain has been confirmed by the IRTF analysis. The thermal properties showed that the copolymerization of PLA on a chitosan’s chain by direct grafting is more thermostable than the one obtained by the ROP method.
From an economic point of view, direct grafting is more suitable for a large industrial scale, because the d, l-lactic acid is far less expensive than the l-lactide. Our method of grafting is more efficient than the one used by [9]. This efficiency is highlighted by the esters’ peak in the IR spectra, which doesn’t exist in their survey. Besides, the solubility test proved that grafted chitosan is neither soluble in the organic solvents of the PLA nor in dilute acid which is a good solvent of pure chitosan. Therefore, due to this insolubility, the PLA-grafted-chitosan will not be used for the next studies on compatibilization of the chitosan/PLA solution.
References
Hsu SC, Don TM, Chiu WY (2002) Synthesis J Appl Polym Sci 86:3047
Yazdani-Pedram M, Lagos Retuert A (2002) Polym Bul 48:93
Ouchi T, Nishizawa H, Ohya Y (1998) Polym 39:5171
Kolhe P, Kannan M R (2003) Biomacromol 4:173
Hu Y, Jiang H, Xu C, Wang Y, Zhu K (2005) Carbohyd Polym 61:472
Huang M, Fang Y (2006) Biopolym 81:160
Prashanth KVH, Tharanathan RN (2003) Carbohyd Polym 54:343
Don TM, King CF, Chiu WY (2002) J Appl Polym Sci 86:3057
Qu X, Wirsén A, Albertson A (1999) J Appl Polym Sci 74:3186
Kim JY, CS Ha, Jo NJ (2002) Polym Int 51:1123
Sakurai K, Maegawa T, Takahashi T (2000) Polym 41:7051
Ding WQ, Lian R, Samuels J, Polk MB (2003) Polymer 44:547
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Suyatma, N.E., Copinet, A., Legin-Copinet, E. et al. Different Pla Grafting Techniques on Chitosan. J Polym Environ 19, 166–171 (2011). https://doi.org/10.1007/s10924-010-0269-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-010-0269-x