Abstract
Continuous vital sign monitoring (CM) may detect ward patient’s deterioration earlier than periodic monitoring. This could result in timely ICU transfers or in a transfer delay due to misperceived higher level of care on the ward. The primary objective of this study was to compare patient’s disease severity upon unplanned ICU transfer, before and after CM implementation. We included a one-year period before and after CM implementation between August 1, 2017 – July 31, 2019. Before implementation, surgical and internal medicine patients’ vital signs were periodically monitored, compared to continuous monitoring with wireless linkage to hospital systems after implementation. In both periods the same early warning score (EWS) protocol was in place. Primary outcome was disease severity scores upon ICU transfer. Secondary outcomes were ICU and hospital length of stay, incidence of mechanical ventilation and ICU mortality. In the two one-year periods 93 and 59 unplanned ICU transfer episodes were included, respectively. Median SOFA (3 (2–6) vs 4 (2–7), p = .574), APACHE II (17 (14–20) vs 16 (14–21), p = .824) and APACHE IV (59 (46–67) vs 50 (36–65), p = .187) were comparable between both periods, as were the median ICU LOS (3.0 (1.7–5.8) vs 3.1 (1.6–6.1), p.962), hospital LOS (23.6 (11.5–38.0) vs 19 (13.9–39.2), p = .880), incidence of mechanical ventilation (28 (47%) vs 22 (54%), p.490), and ICU mortality (11 (13%) vs 10 (19%), p.420). This study shows no difference in disease severity upon unplanned ICU transfer after CM implementation for patients who have deteriorated on the ward.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vital sign deterioration often precedes adverse events on hospital wards and its early detection provides an opportunity to intervene, potentially preventing these events [1,2,3,4,5]. Hospitals have adopted periodic vital sign monitoring and early warning scores (EWS), including Rapid Response Team (RRT) deployment, to improve clinical outcome. The exact added value of these systems to patients’ outcomes remains uncertain [6, 7], presumably due to poor protocol compliance, inaccurate recordings and the periodic nature of the vital sign measurements [8,9,10,11], which can result in clinical deterioration to progress unnoticed [12,13,14]. Continuous vital sign monitoring (CM) on general wards has been advocated to enable early detection of clinical deterioration and to improve patient outcome [15,16,17,18]. In 2010 we introduced a Modified Early Warning Score (MEWS) in our hospital which gradually evolved based on existing EWSs and local expert opinion [19,20,21]. Its final version was implemented in 2014 (Appendix 1). In 2018 continuous monitoring of respiration rate, SpO2, heart rate and blood pressure were introduced on a surgical and internal medicine ward. CM became the basis for the MEWS calculations. Like in previous studies, we recently showed in a before-after study that the implementation of CM on the hospital ward was associated with a significant reduction of unplanned Intensive Care Unit (ICU) transfers (93 vs 59 unique transfers and 84 (3.4%) vs 54 (2.3%) hospital admissions with a transfer (p = 0.03) [19, 22, 23]. CM could indeed have a positive effect in supporting physicians and nurses to be more vigilant and result in earlier detection of clinical deterioration. Its positive effect could prompt interventions, such as timely ICU transfer, resulting in lower disease severity upon ICU arrival.
Opposingly, CM could have a negative effect. Continuous vital sign monitoring could unintentionally prolong the management of deteriorating patients on the ward due to a misperceived higher level of care. CM could cause delayed ICU transfer resulting in higher disease severity upon ICU arrival. The latter has been a major concern of the nursing staff on the ward during implementation of continuous monitoring. The nursing staff feels, and rightfully so, that they are not equipped to care for critically ill patients [24].
Disease severity scores correlate with ICU mortality [25,26,27,28]. Delayed ICU transfer has been associated with increased disease severity scores [29] and increased ICU mortality in critically ill patients [30,31,32]. Whether CM compared to periodic monitoring leads to timely or delayed ICU transfers may be reflected by decreased or increased disease severity scores. Studies that report on the effects of implementation of CM on disease severity at the moment of unplanned ICU transfers are scarce.
In this study we primarily aim to assess the effect of CM on disease severity of patients at the time of unplanned ICU transfer. We hypothesize that disease severity upon ICU transfer, reflected by SOFA and APACHE, does not increase after CM introduction. Second, we aim to assess the effect of CM on the ICU and hospital length of stay, the need for mechanical ventilation and ICU mortality.
Methods
Study design
We performed a before-and-after study in a large tertiary referral hospital in the Netherlands. We included patients who underwent an unplanned transfer from the surgical or internal medicine ward (34 and 26 beds, respectively) to the ICU or Medium Care Unit (MCU). On the surgical ward mainly patients with upper gastrointestinal- and/or hepato-pancreatic-biliary cancer were admitted. General internal medicine, rheumatology, and infectious disease patients were admitted to the internal medicine ward.
Electronic medical record (EMR) notes of the ward and ICU clinicians were reviewed. An unplanned ICU (uICU) transfer was defined as an unforeseen transfer to the ICU or MCU due to clinical deterioration of the patient or after (re)operation for a complication. Ultimately we only included patients who were admitted to the ICU or MCU for more than eight hours to make sure there was enough time to acquire the parameters needed for the calculation of the disease severity scores. The main difference between the ICU and MCU is that the MCU has no mechanical ventilation resources.
We included a one-year period before CM implementation (pre), August 1, 2017 – July 31, 2018, and a one-year period after CM implementation (post), August 1, 2018 – July 31, 2019. The study has been approved by the local ethics committee of the Radboudumc (case number: 2018–4330).
Vital sign measurements and processing
Before CM implementation, nurses manually measured and registered the vital sign parameters in the EMR (Hyperspace, Epic systems corporation, Verona, Wisconsin, USA), using a blood pressure measuring device with a pulse oximeter (heart rate (HR), blood pressure (BP) and oxygen saturation (SpO2)) (Dinamap V100, GE healthcare, Chicago, Illinois, USA or CONTEC 08A, CONTEC Medical Systems Co., Ltd, Qinhuangdao, Hebei, China), an ear thermometer (core temperature (T)) (Covidien Genius 2, Covidien, Dublin, Ireland), and by visual inspection (respiration rate (RR)). In addition to HR, BP, SpO2, RR and T, the supplemental oxygen delivery (L/min) as well as the AVPU score (Alert, Delirious, Voice, Pain or Unresponsive) were collected to acquire a MEWS (Appendix 1). The MEWS is automatically calculated if at least HR, BP, SpO2, RR and T were registered and validated at one timepoint using zero scores for a missing AVPU score and/or missing supplemental oxygen.
We used the same MEWS protocol during both years. This stipulates a vital sign recording and MEWS calculation every 8 h in all patients at baseline. Elevated MEWS (3–5) requires a measurement every 4 h, while hourly measurements and consulting the ward physician or calling the rapid response team (RRT, (intensive care physician and critical care registered nurse)) are mandatory after alarming MEWS (≥ 6).
After CM implementation in august 2018, patients were monitored with a wearable device (VisiMobile, Sotera Wireless, San Diego, CA, USA). This wrist device continuously measures HR, BP, SpO2 and RR. Once every minute, all vital signs were automatically sent to the EMR and were available for periodic validation by the nurses. The MEWS is automatically calculated if HR, BP, SpO2, RR and a manually measured core temperature were registered and validated at one timepoint. Therefore, the used EMR database contained two types of validated vital sign sets 1) sets containing all mandatory vital signs with an automatically calculated MEWS and 2) extra sets without all vital signs that are mandatory for automatic MEWS calculation, resulting in vital sign sets without a MEWS calculation.
Monitors at the nurses’ stations provided clinicians around the clock real-time vital sign data (Appendix 2). The vital sign measurements and trends of the preceding 96 h were also available. Single channel alarms on the monitors or the wrist device were generated when a vital parameter exceeded a preset range (Appendix 3). Patient exclusion or disconnection of the device were only done after clinical assessment by a nurse, or a physician involved in the study. Predefined reasons to disengage CM were a hyperactive delirium during transfer, skin problems and patient’s refusal to be continuously monitored. Patients who underwent elective surgery were connected to the wearable device after surgery.
Data collection for disease severity scores
Data was collected (retrospectively) from the Dutch National Intensive Care Evaluation (NICE) Registry. NICE registry collects data concerning disease severity and outcomes of admissions to the adult ICUs of all Dutch hospitals, with the aim to monitor and improve care. Data of each individual patient was (prospectively) registered by the attending physician. MCU patients are not included in the NICE registry. Data of these patients was retrospectively obtained by EMR review (YE, RP) using the same format as the national registry. The datasets included demographics, transfer diagnoses, comorbidities, length of hospital and ICU stay, vital signs at the time of ICU transfer, ICU interventions, ICU mortality and disease severity scores (SOFA [33], APACHE II [25, 34], and APACHE IV [35]) at the time of ICU transfer.
Study outcome parameters
Primary outcomes were the SOFA, APACHE II and APACHE IV at the moment of the ICU transfer. Secondary outcomes were the length of hospital and ICU stay, the incidence of mechanical ventilation and ICU mortality. Other outcomes were the frequency (times/day) and quality of the validated vital sign sets and time between first alarming MEWS and ICU transfer in the 24 h preceding the ICU admission.
Statistical analysis
Data are presented as mean [SD], median [IQR] or n (%). The Mann–Whitney U test was used to compare non-normally distributed data, the Student’s t-test was used for normally distributed data and the Chi-square was used for categorial variables. A p-value below 0.05 was considered statistically significant. All analyses were performed using statistical package for the social sciences (SPSS) package version 25.0 (SPSS, Inc, Chicago, IL).
Results
Patient characteristics
We identified 136 patients with an unplanned ICU transfer. In the pre period 82 patients underwent a total of 93 uICU transfers and 84 hospital admissions. In the post period 54 patients underwent a total of 59 uICU transfers and 54 hospital admissions. No differences in patient characteristics were found between the groups (Table 1).
Disease severity assessment
91 pre and 56 post uICU transfers were included in the disease severity analysis. Median SOFA was 3 (2–6) vs 4 (2–7), p = 0.574, APACHE II was 17 (14–20) vs 16 (14–21), p = 0.824 and APACHE IV was 59 (46–67) vs 50 (36–65), p = 0.187. There were no significant differences. The corresponding probability scores of ICU mortality are listed in Table 2.
Secondary outcomes
Median ICU LOS was 3.0 (1.7–5.8) vs 3.1 (1.6–6.1) days, p = 0.962, hospital LOS was 23.6 (11.5–38.0) vs 19.0 (13.9–39.2) days, p = 0.880, the incidence of mechanical ventilation was 28 (47%) vs 22 (54%), p.490, and the ICU mortality incidence was 11 (13%) vs 10 (19%), p = 0.420) compared between the pre and post patient population, respectively (Table 3).
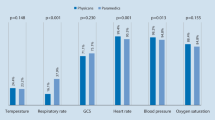
Time between the first alarming MEWS and the uICU transfer itself were comparable between both periods (8.8 h (2.6–18.9) vs 11.9 h (4.5–20.3), p = 0.185). During the 24 h before ICU admissions the nurses’ validation frequency of extra vital sign sets increased after CM implementation (Table 4). Moreover, after implementation of CM these validated extra vital sign sets without a MEWS calculation contained more vital signs than before implementation (systolic blood pressure (51% vs 77%), heart rate (60% vs 79%), respiratory rate (43% vs 75%), oxygen support (49% vs 79%) (Table 4). Comparable patterns were seen during night shifts (Table 4).
Discussion
Continuous vital sign monitoring did not affect the disease severity score of clinically deteriorating patients on a surgical or internal medicine ward at the time of unplanned ICU transfer. CM also had no impact on ICU and hospital length of stay, incidence of mechanical ventilation or ICU mortality. Nurses validated more extra vital signs sets in the EMR and these extra vital sign sets contained also more vital sign parameters after CM implementation. While the nurses’ workflow has been impacted after implementation of CM; our results may suggest that continuous vital sign monitoring neither leads to preemptive nor delayed ICU transfer in clinically deteriorating patients.
Literature pertaining to the impact of CM on decision-making on hospital wards is scarce. Brown et al. reported that implementation of continuous respiratory and heart rate monitoring on a medical-surgical unit was associated with a significant decrease in total length of stay in the hospital and in intensive care unit days for transferred patients, as well as lower code blue rates [36]. They did not observe significant difference in unplanned ICU transfer or in disease severity score upon unplanned ICU arrival. Subbe et al. published a before-and-after study concerning continuous vital sign monitoring in combination with an electronic automated advisory and notification system. They found an association with a reduction in APACHE II scores at the time of ICU transfer [37]. Subbe et al. implied that deployment of the electronic automated advisory and notification system has the ability to significantly increase and expedite rapid response team deployment, which helped to detect clinical deterioration in an earlier phase.
Equivocal results in previous studies focusing on ICU transfer delay and patient outcome are potentially due to differences in study design, setting and definitions of delay. A slow ICU transfer, defined as a transfer 4 h or more after meeting the first physiologic threshold criteria on the ward, was associated with an increased APACHE II score, mortality and costs [29]. Others report an increase in mortality associated with delayed ICU transfer without higher APACHE II and SOFA scores upon ICU transfer [30, 38]. However these studies included patients who clinically deteriorated and were accepted for ICU transfer but stayed on the ward due to shortage in intensive care capacity. These patients were already identified as patients at risk. They probably received maximum treatment on the hospital ward with ICU team involvement, potentially improving physiological parameters and resulting in lower APACHE scores.
In concordance with our findings, previous data from our group also showed that vital sign parameters were frequently lacking in the extra validated vital sign sets [11]. Furthermore, the increased frequency of extra vital sign set validation after CM implementation was also showed by our research group in all patients with CM at the ward [23]. The clinical relevance of the increased validation frequency and vital sign presence of extra vital sign sets after CM implementation has yet to be determined.
In the previous study, we reported a 30 percent reduction in unplanned ICU transfers (93 vs 59 unique transfers and 84 (3.4%) vs 54 (2.3%) hospital admissions with a transfer (p = 0.03) [23]. Therefore, we hypothesize that the availability of continuous monitoring enabled physicians to treat more patients on the hospital ward. Our current study shows no difference in disease severity score upon ICU transfer. Combining our current and previous findings indicate that CM on the ward was successful and obviated the need for ICU transfer of patients, without delaying the transfer of others that eventually needed ICU care.
It is of paramount importance to realize that CM alone is not enough to eliminate ICU transfer delay or expedite the transfers of general ward patients. CM is just one facet of the safety system. Timely ICU transfer will probably require adjustments in RRT protocols, e.g., lower EWS thresholds for calling intensive care staff, as well as revision of ICU transfer and discharge criteria. Future studies are necessary to, for example, evaluate CM in combination with different predefined ICU transfer criteria on patient outcome. Moreover, ICU organization also impacts patient outcome. A closed format ICU staffed with dedicated critical care physicians is associated with lower hospital and ICU mortality and length of stay [39, 40]. We believe that the full potential of any safety system can only be realized when all the participating medical professionals are fully engaged and trained. General guidelines and protocols should always be tailored to the local situation.
Strengths and limitations
Our study has several strengths and limitations. The study was conducted on two different hospital wards with a different patient mix increasing generalizability of the results. However, the single center study design may impede transferability to other hospitals. Another limitation was the retrospective before-and-after study design, which is vulnerable to bias [41]. Therefore a detailed analysis of all the factors that may have affected patient outcome was not possible. To overcome these limitations a multi-center RCT would be necessary to confirm the clinical effectiveness of CM and its effects on disease severity upon ICU transfer. Furthermore, we were not able to analyze the effect of CM on separate parameters of the disease severity scores. For example, Goldhill showed in a retrospective study that in the 24 h prior to ICU transfer only the respiratory parameters in the APACHE score led to a significant increase in disease severity score [5]. We acknowledge that both the SOFA and APACHE scores are not patient-centered outcome parameters. However, we have chosen these as primary outcomes over other parameters such as mortality(ratio) and length of hospital stay since these disease severity scores are calculated with data obtained close to the unplanned ICU transfer. Probably this data reflect the effect of monitoring at the general ward better compared to parameters that are more likely to be affected by factors during the ICU stay [23].
Conclusion
The introduction of continuous vital sign monitoring on the hospital ward did not affect the disease severity of patients at the time of unplanned ICU transfer. This may suggest that continuous monitoring neither results in earlier nor delayed ICU transfer of patients who are clinically deterioration at the ward. The ICU length of stay, hospital length of stay, incidence of mechanical ventilation and ICU mortality were also not affected.
Data availability
On request (corresponding author).
References
Buist MD, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care: A pilot study in a tertiary‐care hospital. Medical Journal of Australia. 1999;171:22–5.
FRANKLIN C, MATHEW J. Developing strategies to prevent inhospital cardiac arrest. Critical Care Medicine. 1994;22:244–7.
Hillman K, Bristow P, Chey T, Daffurn K, Jacques T, Norman S, et al. Duration of life-threatening antecedents prior to intensive care admission. Intensive Care Medicine. 2002;28:1629–34.
Jacques T, Harrison GA, McLaws M-L, Kilborn G. Signs of critical conditions and emergency responses (SOCCER): A model for predicting adverse events in the inpatient setting. Resuscitation. 2006;69:175–83.
Goldhill DR, White SA, Sumner A. Physiological values and procedures in the 24 h before ICU admission from the ward. Anaesthesia. 1999;54:529–34.
Alam N, Hobbelink EL, van Tienhoven AJ, van de Ven PM, Jansma EP, Nanayakkara PWB. The impact of the use of the Early Warning Score (EWS) on patient outcomes: a systematic review. Resuscitation. 2014;85:587–94.
Smith MEB, Chiovaro JC, O’Neil M, Kansagara D, Quiñones AR, Freeman M, et al. Early warning system scores for clinical deterioration in hospitalized patients: A systematic review. Ann Am Thorac Soc. 2014;11:1454–65.
Jones S, Mullally M, Ingleby S, Buist M, Bailey M, Eddleston JM. Bedside electronic capture of clinical observations and automated clinical alerts to improve compliance with an Early Warning Score protocol. Crit Care Resusc. 2011;13:83–8.
Smith AF, Oakey RJ. Incidence and significance of errors in a patient “track and trigger” system during an epidemic of Legionnaires’ disease: retrospective casenote analysis. Anaesthesia. 2006;61:222–8.
Hands C, Reid E, Meredith P, Smith GB, Prytherch DR, Schmidt PE, et al. Patterns in the recording of vital signs and early warning scores: compliance with a clinical escalation protocol. BMJ Quality & Safety. 2013;22:719–26.
Eddahchouri Y, Koeneman M, Plokker M, Brouwer E, van de Belt TH, van Goor H, et al. Low compliance to a vital sign safety protocol on general hospital wards: A retrospective cohort study. Int J Nurs Stud. 2021;115:103849.
Weenk M, Koeneman M, van de Belt TH, Engelen LJLPG, van Goor H, Bredie SJH. Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation. 2019;136:47–53.
Sun Z, Sessler DI, Dalton JE, Devereaux P, Shahinyan A, Naylor AJ, et al. Postoperative Hypoxemia Is Common and Persistent. Anesthesia & Analgesia. 2015;121:709–15.
Turan A, Chang C, Cohen B, Saasouh W, Essber H, Yang D, et al. Incidence, Severity, and Detection of Blood Pressure Perturbations after Abdominal Surgery. Anesthesiology. 2019;130:550–9.
DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, et al. “Identifying the hospitalised patient in crisis”—A consensus conference on the afferent limb of Rapid Response Systems. Resuscitation. 2010;81:375–82.
Vincent JL, Einav S, Pearse R, Jaber S, Kranke P, Overdyk FJ, et al. Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol. 2018.
Downey CL, Chapman S, Randell R, Brown JM, Jayne DG. The impact of continuous versus intermittent vital signs monitoring in hospitals: A systematic review and narrative synthesis. International Journal of Nursing Studies. 2018;84:19–27.
Khanna AK, Ahuja S, Weller RS, Harwood TN. Postoperative ward monitoring – Why and what now? Best Practice & Research Clinical Anaesthesiology. 2019;33:229–45.
Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: A before-and-after concurrence study. Anesthesiology. 2010;112:282–7.
Meynaar IA, van Dijk H, Visser SS, Verheijen M, Dawson L, Tangkau PL. [Rapid response system in derangement of vital signs: five years experience in a large general hospital]. Ned Tijdschr Geneeskd. 2011;155:A3257.
Subbe CP. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94:521–6.
Sun L, Joshi M, Khan SN, Ashrafian H, Darzi A. Clinical impact of multi-parameter continuous non-invasive monitoring in hospital wards: a systematic review and meta-analysis. J R Soc Med. 2020;113:217–24.
Eddahchouri Y, Peelen R v., Koeneman M, Touw HRW, van Goor H, Bredie SJH. Effect of continuous wireless vital sign monitoring on unplanned ICU admissions and rapid response team calls: a before-and-after study. Br J Anaesth. 2022. https://doi.org/10.1016/J.BJA.2022.01.036.
Weenk M, Bredie SJ, Koeneman M, Hesselink G, van Goor H, van de Belt TH. Continuous Monitoring of Vital Signs in the General Ward Using Wearable Devices: Randomized Controlled Trial. Journal of Medical Internet Research. 2020;22:e15471.
KNAUS WA, DRAPER EA, WAGNER DP, ZIMMERMAN JE. APACHE II. Critical Care Medicine. 1985;13:818–29.
Le Gall JR, Loirat P, Alperovitch A. Simplified acute physiological score for intensive care patients. Lancet. 1983.
Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation*. Critical Care Medicine. 2009;37:1649–54.
Vincent J-L, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units. Critical Care Medicine. 1998;26:1793–800.
Young MP, Gooder VJ, McBride K, James B, Fisher ES. Inpatient transfers to the intensive care unit. Journal of General Internal Medicine. 2003;18:77–83.
Cardoso LTQ, Grion CMC, Matsuo T, Anami EHT, Kauss IAM, Seko L, et al. Impact of delayed admission to intensive care units on mortality of critically ill patients: a cohort study. Critical Care. 2011;15:R28.
Bing-Hua YU. Delayed admission to intensive care unit for critically surgical patients is associated with increased mortality. American Journal of Surgery. 2014. https://doi.org/10.1016/j.amjsurg.2013.08.044.
Mokart D, Lambert J, Schnell D, Fouché L, Rabbat A, Kouatchet A, et al. Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leukemia & Lymphoma. 2013;54:1724–9.
Vincent J-L, Moreno R, Takala J, Willatts S, Mendonça A De, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Medicine. 1996;22:707–10.
KNAUS WA, ZIMMERMAN JE, WAGNER DP, DRAPER EA, LAWRENCE DE. APACHE—acute physiology and chronic health evaluation: a physiologically based classification system. Critical Care Medicine. 1981;9:591–7.
Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: Hospital mortality assessment for today’s critically ill patients. Critical Care Medicine. 2006. https://doi.org/10.1097/01.CCM.0000215112.84523.F0.
Brown H, Terrence J, Vasquez P, Bates DW, Zimlichman E. Continuous Monitoring in an Inpatient Medical-Surgical Unit: A Controlled Clinical Trial. The American Journal of Medicine. 2014;127:226–32.
Subbe CP, Duller B, Bellomo R. Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Critical Care. 2017;21:52.
Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit*. Critical Care Medicine. 2007;35:1477–83.
Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician Staffing Patterns and Clinical Outcomes in Critically Ill Patients. JAMA. 2002;288:2151.
MULTZ AS, CHALFIN DB, SAMSON IM, DANTZKER DR, FEIN AM, STEINBERG HN, et al. A “Closed” Medical Intensive Care Unit (MICU) Improves Resource Utilization When Compared with an “Open” MICU. American Journal of Respiratory and Critical Care Medicine. 1998;157:1468–73.
Nedel WL, Silveira F da. Different research designs and their characteristics in intensive care. Revista Brasileira de Terapia Intensiva. 2016;28.
Acknowledgements
We want to thank Sjef van der Velde for contributing to the data curation and providing the database platform.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Financed by Radboud university medical center.
Author information
Authors and Affiliations
Contributions
Yassin Eddahchouri: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Visualization, original draft. Roel Peelen: Conceptualization, Data curation, Writing—review & editing. Mats Koeneman: Formal analysis, Methodology, Project administration, Writing—review & editing. Alec van Veenendaal Conceptualization, Writing—review & editing. Harry van Goor: Conceptualization, Resources, Writing—review & editing. Sebastian JH Bredie: Conceptualization, Resources, Supervision, Writing—review & editing. Hugo Touw: Conceptualization, Methodology, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study has been approved by the local ethics committee of the Radboudumc (case number: 2018–4330). No consent to participate was obliged.
Human and animal ethics
Not applicable.
Consent for publication
Al authors approve the current version of the manuscript and agree to its submission to the Journal of Medical Systems.
Competing interests
All authors declare that they have no competing interest regarding this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eddahchouri, Y., Peelen, R.V., Koeneman, M. et al. The Effect of Continuous Versus Periodic Vital Sign Monitoring on Disease Severity of Patients with an Unplanned ICU Transfer. J Med Syst 47, 43 (2023). https://doi.org/10.1007/s10916-023-01934-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-023-01934-3