Abstract
The stocky skeletons and post-cranial anatomy of many extinct kangaroos indicate that they might have engaged in varied locomotor behaviors, rather than bipedal hopping, as their primary mode of locomotion. This study investigates support for this idea by estimating femoral bone perfusion, which is a correlate of locomotor intensity, in extinct kangaroos compared to living hopping species. Femur blood flow rates can be estimated from the sizes of nutrient foramina on the femur shaft of living and extinct species, without preservation of soft tissue. Estimated femur blood flow rates among the extinct Macropus, Protemnodon and Sthenurinae (Sthenurus, Simosthenurus and Procoptodon) are not significantly different from one another but are significantly greater than in living hopping macropods after accounting for the effect of body mass, consistent with their purportedly different locomotor style. The giant sthenurines have more robust femora than extrapolated from data of living hopping macropods, possibly due to the larger sthenurines requiring relatively stronger leg bones to support their heavier body weights, especially if loaded onto a single limb during bipedal striding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood flow to the femora of terrestrial vertebrates is associated with bone metabolism, because in most cases, regional blood flow rate is determined by regional metabolism (Wolff 2008). Terrestrial vertebrates apply stresses on their leg bones during routine weight-bearing activities, and the frequency of microfractures increases with the intensity of locomotion (Lieberman et al. 2003; Eriksen 2010). Blood flow is required to provide oxygen and metabolic fuel to osteoclasts and osteoblasts to resorb the microfractures and renew the bone tissue (Stabley et al. 2014). Therefore, femur blood flow rate in adult terrestrial vertebrates can provide an index of locomotor activity levels. This argument is supported by the observation that femur blood flow rate and maximum whole-body aerobic metabolic rate during treadmill running exercise scale with body mass with similar exponents in birds and mammals (Seymour et al. 2012; Allan et al. 2014).
More than one-half of total blood flow to the femora is supplied by the nutrient arteries, which penetrate the shaft through nutrient foramina (Trueta 1963). Femur blood flow rates can be estimated from nutrient foramen size because foramen size is influenced by arterial size, which itself is determined by the oxygen demand of the bone tissue (Seymour et al. 2012, 2015). We define femur blood flow rate as perfusion through the principal nutrient artery that penetrates the nutrient foramen (or foramina, if more than one is present) and supplies blood primarily to the bone shaft (diaphysis). Early attempts to estimate femur blood flow rate from nutrient foramen size were based on textbook theory of laminar flow along straight, round tubes according to Poiseuille’s Law, and provided an index of blood flow rate (Qi), then thought to be closely proportional to the absolute flow rate (Seymour et al. 2012). We now know that Poiseuille’s Law is not strictly applicable to the relationship between arterial radius and flow rate (Seymour et al. 2019), and we are beginning to better understand the empirical relationship between arterial radius and foramen radius (Hu et al. 2021a), allowing for more accurate estimates of blood flow rate.
This foramen technique can be applied to extinct vertebrates, providing information on femur blood flow rate and insight into bone metabolism, locomotor activity levels and life history. The utility of this technique should be especially informative when applied to extinct species with obscure or debated lifestyles. Living kangaroos, especially large species, have unique locomotor gaits that include the pentapedal gait, where the forelimbs and tail alternate with the hindlimbs during movement at low speed, and the well-known bipedal hopping gait employed at higher speeds (O'Connor et al. 2014; Thornton et al. 2022). Compared to living species, the locomotion gaits of extinct macropodines (Protemnodon and Macropus) and sthenurines (Sthenurus, Simosthenurus and Procoptodon) are little known and subject to much speculation. Several studies propose that large sthenurines employed bipedal striding, to explain their strikingly more robust skeletal appearance, as well as other post-cranial anatomical features, compared to living kangaroos (Janis et al. 2014; Jones et al. 2021; Wagstaffe et al. 2022). Some Protemnodon may have used quadrupedal movement as one of their gaits, placing more weight on the forelimbs than in living hopping macropods (Janis et al. 2020; Jones et al. 2021). Extinct Macropus such as M. titan could have weighed up to ~175 kg (Wagstaffe et al. 2022), and their heavy body masses might have limited their ability to perform fast bipedal hopping. It is plausible that differences in routine weight-bearing activities and intensity of locomotion may result in differences in leg bone robustness and perfusion rates. This study estimates femur blood flow rate from the sizes of the nutrient foramina in relation to femur morphology and body mass among extinct kangaroos and compares the results with living hopping macropods.
Materials and methods
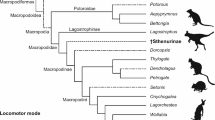
We accept the phylogeny of Macropodiformes as presented in Fig. 1 of Janis et al. (2023). Specimens were sourced from collections at Flinders University and the South Australian Museum. All specimens originated from three sites in South Australia (Naracoorte, Green Water Hole and Lake Callabonna). Measurements were taken from 110 femora (91 individuals) across several genera of fossil kangaroo, including extinct Macropus, Protemnodon, Sthenurus, Simosthenurus and Procoptodon. They were identified by comparing bones with reference collections of skeletons housed at South Australian Museum and Vertebrate Paleontology Laboratory at Flinders University. All fossils belonged to at least one of these genera, as they were collected from the same field sites. However, most had not yet been assigned with museum registration numbers and 26 of them were not yet assigned to a genus and were therefore designated ‘unclassified’ (Online Resource 1). Photographs of the whole fossil femora were taken for future identification. For comparison, 118 femora (59 individuals) from 23 living species of hopping Macropodian macropodoids were selected from the Macropodidae and Potoroidae families. One to four replicates of each living species were selected and measured. For simplicity, we refer to all living species in this study as macropods. All selected living species use some form of saltating (hopping) gait. The living species contain Onychogalea lunata and Notamacropus greyi which persisted in Australia until the 1900s. All the species details are shown in Online Resource 2.
Representative nutrient foramina captured from the femora of a. an extinct Macropus sp. (Group: Extinct Macropus; ID number: 5); b. Protemnodon brehus (Group: Protemnodon; ID number: 2); c. Simosthenurus maddocki (Group: Shenurinae; ID number: 8). The area of the best-fit ellipse around the foramen opening was measured in Fiji. The smallest scale increment is 0.5 mm
Femur length (L, mm) and mid-shaft circumference (C, mm) were measured to 1 mm with a measuring tape, and their ratio (C/L) was calculated to provide a measure of femoral robustness (R). Body masses (Mb, g) of living and extinct animals were estimated from C using a published equation (Helgen et al. 2006). Adult body masses of the living species were also collected from the literature (White and Seymour 2003; Helgen et al. 2006; White et al. 2006) or online sources (animaldiversity.org, eol.org, www.environment.nsw.gov.au, www.gbif.org and nt.gov.au), to compare with the calculated body masses using paired t-test.
Nutrient foramina are usually located at or near the midsection of the femur shafts. Foramen sizes were evaluated with a photographic method and best practices (Hu et al. 2020). A needle tip was used to clean the foramina carefully before taking photographs of each nutrient foramen opening with a scale alongside and level with the opening (Fig. 1). Foramen areas were measured by fitting a best-fit ellipse around the foramen opening in Fiji (open source, www.fiji.sc).
Foramen areas tend to be larger in growing animals presumably due to augmented energy requirements of growth (Hu et al. 2018a, b). Therefore, it is better to exclude juveniles when comparing nutrient foramen sizes among different species. Unfortunately, many of the fossil femora in this study were not assigned to named genera or species, therefore we could not easily determine their life stage. Femur blood flow rates through the nutrient foramina in post-pouch western grey kangaroos including young adults and adults are relatively constant after correcting for body mass (Hu et al. 2018a). Therefore, the age of young adults and adult kangaroos would have little impact on femur blood flow rate. In this study, we reduce the confounding effects of growth by selecting relatively large femora. Sexual dimorphism was not accounted for, as most samples lack information on sex. Femur and foramen data points were averaged from replicates of each living species. However, since there is lack of genera and species information for most extinct specimens, we could only present individual femur and foramen data points for all fossil specimens.
Although textbooks commonly use Poiseuille’s Law to assert that blood flow rate is determined by arterial size, the opposite is in fact true: arterial size is determined by the blood flow rate regime that depends on the oxygen demand of the tissues (Caro et al. 2012), a supply–demand relationship that applies across dynamic, chronic and evolutionary timescales. Furthermore, the relationship between flow rate and arterial size does not strictly conform to the Poiseuille equation (Huo and Kassab 2016). A new empirical relationship between the arterial size and perfusion rate has been determined for mammals: log \(\dot{Q}\) = − 0.20 (log ri) 2 + 1.91 log ri + 1.82, where \(\dot{Q}\) is absolute blood flow rate (ml s−1), and ri is artery lumen radius (cm) (Seymour et al. 2019). To calculate ri from measurements of foramen area, we assume that the artery lumen area is 20% of foramen area, as found in domestic chickens (Hu et al. 2021a). We assume this 20% area proportion is conserved among the endotherms, because the sizes of arteries are always smaller than the sizes of veins that carry the same rate of blood flow but with different pressure gradients. In cases of multiple nutrient arteries on one femur, the summed area of a multiple-foramen femur is not significantly different from the paired single-foramen femur in the same individual (Hu et al. 2021a, b). Therefore, femur \(\dot{Q}\) of a multiple-foramen femur was estimated from the summed foramen areas. If an individual had two femora preserved, femur and foramen data were averaged from both femora, otherwise, one femur remained a datum. In all reported data, \(\dot{Q}\) refers to a single bone blood flow rate.
Allometry studies often involve relating variables (Y) of behavior, anatomy or physiology with the body mass (Mb) of animals. The allometric equation is usually in the form, Y = aMbb, where “a” is the scaling factor, representing the elevation of the allometric relationship, and “b” is the scaling exponent, representing the pattern of the power curve. When the allometric equation is plotted onto log–log axes, the equation becomes logY = loga + blogMb. Scaling relationships of \(\dot{Q}\) or R on Mb (\(\dot{Q}\) = aMbb or R = aMbb) for living hopping macropods and fossil kangaroos were plotted onto graphs with log–log axes. The scaling exponents and scaling factors among the different groups of animals were compared by ANCOVA (Zar 1998) using statistical software (Prism 6.0; GraphPad Software, La Jolla, CA, USA). If exponents between two scaling relationships were different, the Johnson-Neyman test (White 2003) was used to identify the ranges over which the data were significantly different. Fossil femora of known genera were divided into three groups: extinct Macropus (N = 14), Protemnodon (N = 3) and Sthenurinae (N = 48) comprising Sthenurus, Simosthenurus and Procoptodon. Since we could not measure L for some fossil femora due to incomplete or damaged specimens, we do not always present L and R values (Online Resource 2). Scaling relationships of \(\dot{Q}\) or R on Mb were also compared among the three extinct groups to test for any significant differences.
Results
Femur blood flow rate (\(\dot{Q}\))
Estimated body masses (Mb) of the living hopping macropods range from 1.35 to 43.9 kg, which represents a 32.4-fold range. The estimated Mb values are similar to those collected from the literature (paired t-test, p = 0.95). Estimated Mb of fossil kangaroos range from 9.08 to 183.2 kg, which represents a 20.2-fold range. Scaling of estimated femur \(\dot{Q}\) on Mb in living hopping macropods and among the extinct kangaroos is shown in Fig. 2 along with their scaling equations. The group of living species has an exponent of 0.73 ± 0.28 (95% confidence interval), which is not significantly different from the 0.57 ± 0.38 found collectively across all extinct kangaroos (F1, 110 = 0.92, p = 0.34). However, the scaling factors are significantly different (F1, 111 = 21.4, p < 0.0001), with \(\dot{Q}\) in extinct kangaroos being significantly greater than in living hopping macropods. If unclassified individuals were removed, scaling exponents between the living species and extinct individuals remain similar (F1, 85 = 0.58, p = 0.45) while the scaling factors remain significantly different (F1, 86 = 11.9, p = 0.0009).
Scaling of estimated femur blood flow rate (\(\dot{Q}\)) on body mass in living hopping macropods and extinct kangaroos. Data are on log–log axes. The allometric equations for femur blood flow rate (\(\dot{Q}\); ml s−1) on body mass (Mb; g) are \(\dot{Q}\) = 4.50 × 10–6 Mb0.73±0.28 (95%CI) for 23 species of living hopping macropods, and \(\dot{Q}\) = 5.48 × 10–5 Mb0.57±0.38 for 91 fossil kangaroo individuals, including 14 extinct Macropus, 48 Sthenurinae, three Protemnodon and 26 unclassified individuals. The dotted lines delineate the 95% confidence intervals for each regression mean
Examining only the three groups of extinct kangaroos shows that the scaling of \(\dot{Q}\) in Sthenurinae is not significantly different from the extinct Macropus and Protemnodon groups (F2, 59 = 0.57, p = 0.57 for scaling exponent; F2, 61 = 0.11, p = 0.89 for scaling factor) (Online Resource 3). The scaling exponent of 0.73 ± 0.28 for living hopping macropods is not significantly different from that (0.35 ± 0.56) of extinct Macropus (F1, 33 = 1.72, p = 0.20) or Sthenurinae (0.61 ± 0.29) (F1, 67 = 0.40, p = 0.53). However, the scaling factor for the living hopping macropods is significantly lower than the extinct Macropus (F1, 34 = 11.88, p = 0.0015) and Sthenurinae (F1, 68 = 11.2, p = 0.0014). Protemnodon has only three data points, so its scaling relationship is yet to be established. However, they all sit closely with the two other extinct groups, implying similar \(\dot{Q}\) (Online Resource 3).
Femur robustness (R)
Femur robustness (R = C/L) was calculated for all living hopping macropod species used in this study, as well as in six extinct Macropus, 30 sthenurines and a single Protemnodon. The scaling of R on Mb across 23 living hopping macropod species and six extinct Macropus individuals exhibit significantly different scaling exponents (living hopping macropods: 0.02 ± 0.02; extinct Macropus: 0.13 ± 0.13) (F1, 25 = 5.28, p = 0.030) (Fig. 3). R in extinct Macropus increases rapidly compared to R in living species. The Johnson-Neyman test indicates that extinct Macropus weighing over 39.2 kg had more robust femora than the living species. Compared to the living hopping macropod species, R on Mb in the 30 sthenurines has a significantly steeper scaling exponent of 0.14 ± 0.06 (F1, 49 = 41.6, p < 0.0001). The Johnson-Neyman test indicates that sthenurines weighing over 18.3 kg had significantly more robust femora than predicted by living hopping macropods. Comparison of R on Mb between the Sthenurinae and extinct Macropus shows statistically indistinguishable scaling exponents (F1, 32 = 0.048, p = 0.83), but significantly different scaling factors (F1, 33 = 16.1, p = 0.0003). The scaling of both the living hopping macropods and extinct Macropus have scaling exponents that are not significantly different from 0, whereas Sthenurinae has a scaling exponent that is significantly steeper than 0 (Fig. 3).
Scaling of femur robustness on body mass in living hopping macropods and extinct kangaroos. Data are on log–log axes. The allometric equations for femur robustness (R; ratio of femur mid-shaft circumference and length) on body mass (Mb; g) are R = 0.22Mb0.02±0.02 (95%CI) for 23 living hopping macropod species, R = 0.74Mb0.13±0.13 for six extinct Macropus individuals, and R = 0.07Mb0.14±0.03 for 30 Sthenurinae individuals. The single Protemnodon is superimposed only. The dotted lines delineate the 95% confidence intervals for each regression mean
Discussion
Most living kangaroos use bipedal hopping for fast locomotion, which is a very efficient gait, especially in larger macropods at higher speeds (Dawson and Taylor 1973; Baudinette et al. 1992; Thornton et al. 2022). The energy savings provided by the gastrocnemius and plantaris tendons of the hindlimb is affected by body mass and locomotion speed, such that larger species undertaking fast hopping tend to show the greatest energy savings (Baudinette et al. 1992). Although bipedal hopping is energy-efficient in large living kangaroos, hopping presents a biomechanical challenge to very large kangaroos. The gastrocnemius tendon cross sectional area does not scale in proportion to peak force loads with increasing body mass, imposing a theoretical maximum body mass of 50–150 kg for a hopper based on analyses of living hopping macropods (Bennett and Taylor 1995; McGowan et al. 2008; Snelling et al. 2017). Above this body mass, the gastrocnemius tendon safety factor drops below 1, and the tendon is at risk of rupturing during fast or accelerative hopping (McGowan et al. 2008; Snelling et al. 2017). Some extinct kangaroo species likely weighed more than this maximum theoretical body mass. They might not have hopped like a living kangaroo and may have instead used different locomotion gaits, such as a bipedal striding gait and a quadrupedal gait (Wells and Tedford 1995; Janis et al. 2014, 2020, 2023; Jones et al. 2021; Wagstaffe et al. 2022). Although it is difficult to imagine a striding gait in recent kangaroos, red kangaroos (Macropus rufus) exhibit it during swimming (Wilson 1974).
If extinct large kangaroos had different gaits, it might be reflected in the structure and function of the hind limb bones. To reduce the torque of ground reaction force and improve the effective mechanical advantage around the joints of the limbs, placental mammals transition to more upright limb postures as body mass increases (Biewener 2005). In contrast, the hindlimbs retain a crouched posture across different sizes of the living macropods, because hopping apparently requires this posture to stretch the tendons on landing in preparation for the next jump (McGowan et al. 2008; Snelling et al. 2017). Hopping might still be one of the potential gaits for large-bodied extinct Macropus, although they would have likely had to compensate for higher forces with thicker tendons and more robust leg bones. Indeed, large living hopping kangaroos tend to have longer calcaneal tuberosity with thickened cortical bone structure (Wagstaffe et al. 2022; Janis et al. 2023). Large-bodied extinct M. titan had calcaneal tuberosity length similar to smaller macropodians after correcting for body mass, and they had calcanei with thickened cortical bone (Wagstaffe et al. 2022; Janis et al. 2023), implying their potential ability to engage in bipedal hopping. However, the generally more robust and rigid skeletons of sthenurines have been interpreted as adaptations to bipedal striding (Janis et al. 2014; Jones et al. 2021; Wagstaffe et al. 2022). Calcaneal tubers of sthenurines were less resistant and their long foot bones were more resistant to medial bending stresses compared to Macropus (Wagstaffe et al. 2022), indicating that sthenurines had difficulty performing bipedal hopping. Compared to M. giganteus, the femora of S. stirlingi and S. tindalei have a relatively longer greater trochanter, which is more closely aligned with the axis of the femur shaft (Wells and Tedford 1995). The greater trochanter is the attachment point for the gluteal muscles, and the likely larger gluteal muscles may have assisted with support while placing the whole-body weight on one leg during bipedal striding (Aiello and Dean 1990; Janis et al. 2014). Direct evidence of bipedal striding comes from separate footprints in a recently discovered fossil trackways in the Pliocene of Central Australia (Camens and Worthy 2019). In addition, sthenurines were probable browsers, as their forearm morphology overlaps with that of extant arboreal taxa (Jones et al. 2021), and it does not appear to be a weight bearing limb (Janis et al. 2020). As such, more robust femora were probably necessary for sthenurines, especially large-bodied individuals, to withstand the increased forces placed on single limbs during bipedal striding locomotion.
Unlike living kangaroos, which mostly use bipedal hopping as one of their primary locomotor gaits, the present study supports the hypothesis that extinct kangaroos used different modes of locomotion than extant ones because there is a disjunction in the scaling relationships between robustness (R) and femur blood flow rate (\(\dot{Q}\)) in relation to body mass. Extinct Macropus tended to have R higher than that of the living hopping macropods as their body masses increases beyond 39.2 kg, which is close to the theoretical optimal body mass for effective hopping (~ 35 kg) (Bennett 2000). Most living species of Macropus and Osphranter are above this optimal body mass (Bennett 2000). Consequently, they may have evolved elongate tibiae to uphold tendon energy storage and avoid tendon rupture during rapid hopping (Janis et al. 2023). The scaling of R on Mb in both the living hopping macropods and extinct Macropus have exponents that are not significantly different from 0 (Fig. 3), revealing that R changes little with body mass, at least in these two groups. Similarly, all hindlimb external diameters scale isometrically with bone length in macropodoids (McGowan et al. 2008). This constant robustness is different from the positive exponents found generally in mammals (Christiansen 1999) and cursorial birds (Hu et al. 2023). Despite the shallow scaling exponent of R in living hopping macropods and fossil Macropus being essentially independent of Mb, R is greater among sthenurines, and the scaling exponent rises to 0.14 ± 0.03 (Fig. 3). This high exponent implies that sthenurines had distinct bone morphologies, which are likely influenced by their special standing postures and locomotor gaits, possibly including bipedal striding.
Blood flow to bones is determined by the metabolic requirements of the bone tissue, so it should be related to bone volume and the stresses placed on bones that cause microfractures requiring repair. If extinct kangaroos moved like modern large kangaroos or had very similar saltatory gaits, we would expect their \(\dot{Q}\) to be similar to living hopping macropod species of the same body mass. However, the elevated \(\dot{Q}\) (Fig. 2) suggests that it might not be the case. The higher femur \(\dot{Q}\) in extinct kangaroos appears to be directly functionally associated with their more robust femora. A larger femur requires a higher blood perfusion rate to support and maintain bone structural integrity. The increase of R is not the only source of the elevated \(\dot{Q}\) in extinct kangaroos, as the scaling patterns of R on Mb are different from the scaling patterns of \(\dot{Q}\) on Mb among different groups (Fig. 3, Online Resource 3). \(\dot{Q}\) is influenced by metabolic demand of the femora, so it is also related to energy required for repairing microfractures caused by locomotor activities (Seymour et al. 2012; Allan et al. 2014).
Estimated femur \(\dot{Q}\) scales with Mb raised to an exponent of 0.57 ± 0.38 among extinct kangaroos, which is not significantly different from the living hopping macropod species (0.73 ± 0.28) (Fig. 2) nor is it different from the scaling exponent (0.80 ± 0.06) for maximum oxygen consumption rate in seven species of adult hopping macropods (Frappell and Baudinette 1995). However, extinct kangaroos had significantly higher estimated \(\dot{Q}\) than in living macropods, even after accounting for differences in body mass. Although there are only three \(\dot{Q}\) data for Protemnodon in this study, they all sit closely to the other extinct kangaroos, also indicating a higher \(\dot{Q}\) compared to the living species. The higher \(\dot{Q}\) in extinct kangaroos was possibly due to their different locomotor styles compared to the living hopping macropods, and their locomotor behaviors may have caused higher incidence of microfractures during daily activities or their locomotor behaviors were not as energy efficient as the living hopping macropods, thus requiring greater perfusion of the femoral bone tissue.
The \(\dot{Q}\) and R differences between the groups in the present study imply that the extinct kangaroos, including the large extinct Macropus, did not locomote like living hopping kangaroos. Although it is difficult to assign specific locomotor gaits to different extinct kangaroo groups with knowledge only of \(\dot{Q}\) and R values, our results indicate that they required a larger amount of femur perfusion in more robust leg bones. Many previous studies have provided evidence that extinct kangaroos utilize varied locomotor behaviors. It has been reported that some large extinct Macropus had similarly robust tibia as the other large extinct kangaroos, and some of their bones cluster with living kangaroos, while others cluster with large sthenurines on multivariate plots (Janis et al. 2014). Although sthenurines might have used bipedal striding, it remains possible that they could have utilized hopping as a secondary gait. Nonetheless, most sthenurines and large Protemnodon species having shorter tibiae, which reduces the possibility for bipedal hopping (Janis et al. 2023). The smaller-sized sthenurines, Hadronomas puckridgi and Sthenurus andersoni, had similar tibia lengths to macropodines with similar body masses, while some large-sized sthenurines (e.g. Procoptodon gilli) had shorter tibia lengths (Janis et al. 2023). Indeed, Janis et al. (2014) proposed that smaller sthenurines could have used hopping at fast speed and bipedal striding only at slow speeds, while large species may have used bipedal striding at all speeds. If large-bodied sthenurines utilize bipedal striding at high speed, ground reaction forces applied to each leg would be very large during fast movement, possibly accounting for the elevated \(\dot{Q}\) and the higher scaling exponent of R on Mb in sthenurines. Although R of Protemnodon also seems to be elevated similarly to sthenurines (Fig. 3), it is not possible to draw any definitive conclusions with only one data point. For a better understanding of Protemnodon locomotor behaviors, further research is required.
Primary gaits have been suggested to be variable within the genus of Protemnodon, ranging from bipedal hopping to quadrupedal bounding, as some species had femoro-tibial indices (ratio of the limb elements and related to different locomotor gaits) similar to living Macropus, while some others were similar to tree kangaroos (Kear et al. 2008). The long tibia in certain Protemnodon species (e.g. P. senus) seems to correlate with their apparently frequent browsing behaviour, rather than being related to bipedal hopping locomotion (Janis et al. 2023). Trackways of Protemnodon also showed that they performed saltatory gait (Carey et al. 2011). The varied and inconsistent bone morphologies in the extinct kangaroos may be related to different habitat and life history characteristics among the different species. Therefore, compared to the living species, locomotor behaviors may be more varied among the extinct species, or even within the same extinct genera. As suggested by Janis et al. (2023), bipedal hopping was probably one of the many locomotor gaits that kangaroos use. What makes living hopping kangaroos special is the absence of other species that had a wider range of locomotory adaptations.
Conclusions
In summary, femur blood flow rate of extinct kangaroos was higher than that in living hopping macropods, indicating a greater amount energy was required during routine locomotor activities. Furthermore, the higher bone perfusion may also reflect their more robust bones in relation to their different locomotor behaviors, compared to living hopping macropods. The higher blood flow rate and more robust femora in sthenurines compared to living hopping macropods suggests they likely employed a special locomotor gait involving greater forces applied to the leg bones during locomotion, possibly bipedal striding.
References
Aiello L, Dean C (1990) An Introduction to Human Evolutionary Anatomy. Academic Press, London
Allan GH, Cassey P, Snelling EP, Maloney SK, Seymour RS (2014) Blood flow for bone remodelling correlates with locomotion in living and extinct birds. J Exp Biol 217(16):2956–2962. https://doi.org/10.1242/jeb.102889
Baudinette R, Snyder GK, Frappell PB (1992) Energetic cost of locomotion in the tammar wallaby. Am J Physiol 262(5):R771–R778. https://doi.org/10.1152/ajpregu.1992.262.5.R771
Bennett M (2000) Unifying principles in terrestrial locomotion: do hopping Australian marsupials fit in? Physiol Biochem Zool 73(6):726–735. https://doi.org/10.1086/318110
Bennett M, Taylor G (1995) Scaling of elastic strain energy in kangaroos and the benefits of being big. Nature 378:56–59. https://doi.org/10.1038/378056a0
Biewener AA (2005) Biomechanical consequences of scaling. J Exp Biol 208(9):1665–1676. https://doi.org/10.1242/jeb.01520
Camens A, Worthy T (2019) Walk like a kangaroo: new fossil trackways reveal a bipedally striding macropod in the Pliocene of Central Australia. J Vertebr Paleontol SVP Prog Abstr Book 2019:72
Carey SP, Camens AB, Cupper ML, Grün R, Hellstrom JC, McKnight SW, Mclennan I, Pickering DA, Trusler P, Aubert M (2011) A diverse Pleistocene marsupial trackway assemblage from the Victorian Volcanic Plains, Australia. Quat Sci Rev 30(5–6):591–610. https://doi.org/10.1016/j.quascirev.2010.11.021
Caro CG, Pedley T, Schroter R, Seed W (2012) The Mechanics of the Circulation. Cambridge University Press, Cambridge
Christiansen P (1999) Scaling of mammalian long bones: small and large mammals compared. J Zool 247(3):333–348. https://doi.org/10.1111/j.1469-7998.1999.tb00996.x
Dawson, TJ, Taylor CR (1973) Energetic cost of locomotion in kangaroos. Nature 246(5431):313–314.
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 11:219–227. https://doi.org/10.1007/s11154-010-9153-1
Frappell P, Baudinette R (1995) Scaling of respiratory variables and the breathing pattern in adult marsupials. Respir Physiol 100(1):83–90. https://doi.org/10.1016/0034-5687(94)00122-G
Helgen KM, Wells RT, Kear BP, Gerdtz WR, Flannery TF (2006) Ecological and evolutionary significance of sizes of giant extinct kangaroos. Aust J Zool 54(4):293–303. https://doi.org/10.1071/ZO05077
Hu Q, Nelson TJ, Snelling EP, Seymour RS (2018a) Femoral bone perfusion through the nutrient foramen during growth and locomotor development of western grey kangaroos (Macropus fuliginosus). J Exp Biol 221(4):jeb168625. https://doi.org/10.1242/jeb.168625
Hu Q, Nelson TJ, Snelling EP, Seymour RS (2018b) Correction: Femoral bone perfusion through the nutrient foramen during growth and locomotor development of western grey kangaroos (Macropus fuliginosus). J Exp Biol 221(13):jeb188029. https://doi.org/10.1242/jeb.188029
Hu Q, Nelson TJ, Seymour RS (2020) Bone foramen dimensions and blood flow calculation: best practices. J Anat 236(2):357–369. https://doi.org/10.1111/joa.13106
Hu Q, Nelson TJ, Seymour RS (2021a) Morphology of the nutrient artery and its foramen in relation to femoral bone perfusion rates of laying and non‐laying hens. J Anat 240(1):94–106. https://doi.org/10.1111/joa.13535
Hu Q, Nelson TJ, Seymour RS (2021b) Regional femoral bone blood flow rates in laying and non-laying chickens estimated with fluorescent microspheres. J Exp Biol 224(16):jeb242597. https://doi.org/10.1242/jeb.242597
Hu Q, Miller CV, Snelling EP, Seymour RS (2023) Blood flow rates to leg bones of extinct birds indicate high levels of cursorial locomotion. Paleobiology 49(4):700–711. https://doi.org/10.1017/pab.2023.14
Huo Y, Kassab GS (2016) Scaling laws of coronary circulation in health and disease. J Biomech 49(12):2531–2539. https://doi.org/10.1016/j.jbiomech.2016.01.044
Janis CM, Buttrill K, Figueirido B (2014) Locomotion in extinct giant kangaroos: were sthenurines hop-less monsters? Plos One 9(10):e109888. https://doi.org/10.1371/journal.pone.0109888
Janis CM, Napoli JG, Billingham C, Martín-Serra A (2020) Proximal humerus morphology indicates divergent patterns of locomotion in extinct giant kangaroos. J Mamm Evol 27(4):627–647. https://doi.org/10.1007/s10914-019-09494-5
Janis CM, O’Driscoll AM, Kear BP (2023) Myth of the QANTAS leap: perspectives on the evolution of kangaroo locomotion. Alcheringa 47(4):671–685. https://doi.org/10.1080/03115518.2023.2195895
Jones B, Martín-Serra A, Rayfield EJ, Janis CM (2021) Distal humeral morphology indicates locomotory divergence in extinct giant kangaroos. J Mamm Evol 29(1):27–41. https://doi.org/10.1007/s10914-021-09576-3
Kear BP, Lee MS, Gerdtz WR, Flannery TF (2008) Evolution of hind limb proportions in kangaroos (Marsupialia: Macropodoidea). In: Sargis EJ, Dagosto M (eds) Mammalian Evolutionary Morphology: A Tribute to Fred Szalay. Springer, Dordrecht, Netherlands, pp 25–35
Lieberman DE, Pearson OM, Polk JD, Demes B, Crompton AW (2003) Optimization of bone growth and remodeling in response to loading in tapered mammalian limbs. J Exp Biol 206(18):3125–3138. https://doi.org/10.1242/jeb.00514
McGowan CP, Skinner J, Biewener AA (2008) Hind limb scaling of kangaroos and wallabies (superfamily Macropodoidea): implications for hopping performance, safety factor and elastic savings. J Anat 212(2):153–163. https://doi.org/10.1111/j.1469-7580.2007.00841.x
O'Connor SM, Dawson TJ, Kram R, Donelan JM (2014) The kangaroo's tail propels and powers pentapedal locomotion. Biol Lett 10(7):20140381. https://doi.org/10.1098/rsbl.2014.0381
Seymour RS, Smith SL, White CR, Henderson DM, Schwarz-Wings D (2012) Blood flow to long bones indicates activity metabolism in mammals, reptiles and dinosaurs. Proc R Soc B: Biol Sci 279(1728):451–456. https://doi.org/10.1098/rspb.2011.0968
Seymour RS, Angove SE, Snelling EP, Cassey P (2015) Scaling of cerebral blood perfusion in primates and marsupials. J Exp Biol 218(16):2631–2640. https://doi.org/10.1242/jeb.124826
Seymour RS, Hu Q, Snelling EP, White CR (2019) Interspecific scaling of blood flow rates and arterial sizes in mammals. J Exp Biol 222(7):jeb199554. https://doi.org/10.1242/jeb.199554
Snelling EP, Biewener AA, Hu Q, Taggart DA, Fuller A, Mitchell D, Maloney SK, Seymour RS (2017) Scaling of the ankle extensor muscle‐tendon units and the biomechanical implications for bipedal hopping locomotion in the post‐pouch kangaroo Macropus fuliginosus. J Anat 231(6):921–930. https://doi.org/10.1111/joa.12715
Stabley JN, Moningka NC, Behnke BJ, Delp MD (2014) Exercise training augments regional bone and marrow blood flow during exercise. Med Sci Sports Exerc 46(11):2107–2112. https://doi.org/10.1249/MSS.0000000000000342
Thornton LH, Dick TJ, Bennett MB, Clemente CJ (2022) Understanding Australia’s unique hopping species: a comparative review of the musculoskeletal system and locomotor biomechanics in Macropodoidea. Aust J Zool 69(4):136–157. https://doi.org/10.1071/ZO21048
Trueta J (1963) The role of the vessels in osteogenesis. J Bone Joint Surg 45B(2):402–418.
Wagstaffe AY, O'Driscoll AM, Kunz CJ, Rayfield EJ, Janis CM (2022) Divergent locomotor evolution in “giant” kangaroos: evidence from foot bone bending resistances and microanatomy. J Morphol 283(3):313–332. https://doi.org/10.1002/jmor.21445
Wells RT, Tedford RH (1995) Sthenurus (Macropodidae, Marsupialia) from the Pleistocene of Lake Callabonna, South Australia. Bull Am Mus Nat Hist 225:1–111.
White CR (2003) Allometric analysis beyond heterogeneous regression slopes: use of the Johnson-Neyman technique in comparative biology. Physiol Biochem Zool 76(1):135–140. https://doi.org/10.1086/367939.
White CR, Seymour RS (2003) Mammalian basal metabolic rate is proportional to body mass2/3. Proc Natl Acad Sci U S A 100(7):4046–4049. https://doi.org/10.1073/pnas.0436428100
White CR, Phillips NF, Seymour RS (2006) The scaling and temperature dependence of vertebrate metabolism. Biol Lett 2(1):125–127. https://doi.org/10.1098/rsbl.2005.0378
Wilson GR (1974) How kangaroos swim. Search 6(11):598–600.
Wolff CB (2008) Normal cardiac output, oxygen delivery and oxygen extraction. Adv Exp Med Biol 599:169–182.
Zar JH (1998) Biostatistical Analysis. Prentice-Hall, Inc, New Jersey
Acknowledgements
We thank Mary-Anne Binnie from South Australian Museum for providing access to the extinct kangaroo specimens.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This study was supported by Australian Research Council (grant no. DP 170104952).
Author information
Authors and Affiliations
Contributions
Q.H. and R.S.S contributed to the study design and data collection. Q.H. analyzed the data, conducted literature review, generated figures and tables, and wrote the first draft of the manuscript. E.P.S. and R.T.W provided valuable insights and contributed to manuscript proofreading. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, Q., Seymour, R.S., Snelling, E.P. et al. Blood flow rate to the femur of extinct kangaroos implies a higher locomotor intensity compared to living hopping macropods. J Mammal Evol 31, 2 (2024). https://doi.org/10.1007/s10914-023-09701-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s10914-023-09701-4