Abstract
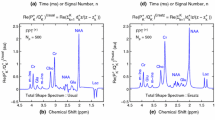
Brain tumors are the leading cause of cancer-related deaths among children. Increasing attention in pediatric neuro-oncology has been given to magnetic resonance spectroscopy (MRS). Notwithstanding the important achievements, the potential of MRS for pediatric neuro-oncology is yet to be realized. This is largely due to reliance upon inadequate signal processing methods based upon the fast Fourier transform (FFT) plus fitting. Herein, we applied an advanced signal processor, the fast Padé transform (FPT) to MRS time signals encoded in vivo from a glioma in a pediatric patient, using a 1.5T scanner. Three echo times (TE) were used: 22, 136 and 272 ms. Compared to those from the FFT, the total shape spectra from the FPT were better resolved. The most striking advantages of the FPT lie in its parametric capabilities from which component spectra were generated. At the shortest TE, for which spectral density is greatest, the FPT resolved the numerous overlapping resonances, delineating myoinositol and other short-lived metabolites. The FPT resolved components of diagnostically-important peaks centered at chemical shifts near 2.0, 3.0 and 3.2 parts per million. The latter includes not only free choline, but also the cancer biomarker, phosphocholine. An information-preserving procedure for suppression of residual water is introduced and validated, via windowing using a step function. This investigation demonstrates that mathematical optimization through the FPT can be successfully applied to MRS time signals encoded in vivo from pediatric brain tumors using standard clinical scanners at 1.5T. Improved diagnostic yield within pediatric neuro-oncology is anticipated thereby.

















Similar content being viewed by others
Notes
Note that Glx is often used as a joint acronym for both Glu and Gln.
Abbreviations
- Ace:
-
Acetate
- Ala:
-
Alanine
- Asp:
-
Aspartate
- ARMA:
-
Autoregressive moving average
- bl:
-
Band limited
- BW :
-
Bandwidth
- Cho:
-
Choline
- Cr:
-
Creatine
- DFT:
-
Discrete Fourier transform
- DWI:
-
Diffusion weighted imaging
- FFT:
-
Fast Fourier transform
- FID:
-
Free induction decay
- fMRI:
-
Functional MRI
- FPT:
-
Fast Padé transform
- FWHM:
-
Full width at half maximum
- GABA:
-
Gamma amino butyric acid
- GE:
-
General Electric
- Gln:
-
Glutamine
- Glu:
-
Glutamate
- Glx:
-
Glutamine plus glutamate
- HLSVD:
-
Hankel–Lanczos singular value decomposition
- IDFT:
-
Inverse discrete Fourier transform
- IFFT:
-
Inverse fast Fourier transform
- Lac:
-
Lactate
- Leu:
-
Leucine
- Lip:
-
Lipids
- m-Ins:
-
Myoinositol
- MR :
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- MRSI:
-
Magnetic resonance spectroscopic imaging
- ms :
-
Milliseconds
- NAA:
-
N-acetyl aspartate
- NAAG:
-
N-acetyl aspartyl glutamic acid
- PC:
-
Phosphocholine
- PCM:
-
Personalized cancer medicine
- PCr:
-
Phosphocreatine
- PRESS:
-
Point-resolved spectroscopy sequence
- ppm:
-
Parts per million
- rad:
-
Radian
- s-Ins:
-
Scylloinositol
- SNR:
-
Signal-noise ratio
- SNS:
-
Signal-noise separation
- SRI:
-
Spectral range of interest
- SVD:
-
Singular value decomposition
- Tau:
-
Taurine
- TE:
-
Echo time
- TR:
-
Repetition time
- Val:
-
Valine
References
L. Brandão, T.Y. Poussaint, Pediatric brain tumors. Neuroimaging Clin. N. Am. 23, 499–525 (2013)
J. Crawford, Childhood brain tumors. Pediatr. Rev. 34, 63–78 (2013)
E. Bouffet, U. Tabori, A. Huang, U. Bartels, Possibilities of new therapeutic strategies in brain tumors. Cancer Treat. Rev. 36, 335–341 (2010)
F.S. Davis, Epidemiology of brain tumors. Expert Rev. Anticancer Ther. 7(Suppl. 12), S3–S6 (2007)
J. Fisher, J.A. Schwartzbaum, M. Wrensch, J. Wiemels, Epidemiology of brain tumors. Neurol. Clin. 25, 867–890 (2007)
M.J. Paldino, E.N. Faerber, T.Y. Poussaint, Imaging tumors of the pediatric central nervous system. Radiol. Clin. N. Am. 49, 589–616 (2011)
A.E. Li, D.A. Bluemke, Magnetic resonance imaging, in Cancer Principles & Practice of Oncology, 6th edn., ed. by V.T. de Vita, S. Hellman, S.A. Rosenberg (Lippincott Williams & Wilkins, Philadelphia, 2001), pp. 669–679
S. Gudowius, V. Engelbrecht, M. Messing-Junger, G. Reifenberger, J. Gärtner, Diagnostic difficulties in childhood bilateral thalamic astrocytomas. Neuropediatrics 33, 331–335 (2002)
Dž Belkić, K. Belkić, Signal Processing in Magnetic Resonance Spectroscopy with Biomedical Applications (Taylor & Francis, London, 2010)
P. Svolos, E. Kousi, E. Kapsalaki, K. Theodorou, I. Fezoulidis, C. Kappas, I. Tsougos, The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: a review and future perspectives. Cancer Imaging 14, 20 (2014)
K. Sartor, M. Hartmann, J. Fiebach, I. Harting, T. Wilhelm, S. Heiland, Normal and abnormal water diffusion in the brain. Rofo. Fortschr. Geb. Rontgenstr. Neuen Bildgeb. Verfahr. 175, 1317–1329 (2003)
A. Horská, D.D.M. Lin, MRI of the brain, in Magnetic Resonance Imaging and Spectroscopy, Volume 3 of Comprehensive Biomedical Physics, ed. by Dž. Belkić, K. Belkić (Elsevier, Amsterdam, 2014), pp. 99–114
M. Nakaiso, M. Uno, M. Harada, T. Kageji, O. Takimoto, S. Nagahiro, Brain abscess and glioblastoma identified by combined proton magnetic resonance spectroscopy and diffusion-weighted magnetic resonance imaging—two case reports. Neurol. Med. Chir. (Tokyo) 42, 346–348 (2002)
A.S. Bick, N. Levin, G. Goelman, Functional magnetic resonance imaging (fMRI), in Magnetic Resonance Imaging and Spectroscopy, Volume 3 of Comprehensive Biomedical Physics, ed. by Dž. Belkić, K. Belkić (Elsevier, Amsterdam, 2014), pp. 69–80
A. Ramos, A. Hilario, A. Lagares, E. Salvador, A. Perez-Nuñez, J. Sepulveda, Brainstem gliomas. Semin. Ultrasound CT MRI 34, 104–112 (2013)
S.J. Nelson, Multivoxel magnetic resonance spectroscopy of brain tumors. Mol. Cancer Ther. 2, 497–507 (2003)
L.A. Brandão, R.C. Domingues, MR Spectroscopy of the Brain (Lippincott Williams & Wilkins, Philadelphia, 2004)
P.E. Sijens, M. Oudkerk, \(^{1}\)H chemical shift imaging characterization of human brain tumor and edema. Eur. Radiol. 12, 2056–2061 (2002)
W. Möller-Hartmann, S. Herminghaus, T. Krings, G. Marquardt, H. Lanfermann, U. Pilatus, F. Zanella, Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology 44, 371–381 (2002)
A. Panigrahy, M.D. Krieger, I. Gonzalez-Gomez, X. Liu, J.G. McComb, J.L. Finlay, M.D. Nelson, F.H. Gilles, S. Blüml, Metabolism of diffuse intrinsic brainstem gliomas in children. Neuro Oncol. 10, 32–44 (2008)
L. Porto, M. Kieslich, K. Franz, T. Lehrnbecher, F. Zanella, U. Pilatus, E. Hattingen, MRS differentiation between high and low grade astrocytomas: comparison between paediatric and adult tumours. Eur. J. Paediatr. Neurol. 15, 214–221 (2011)
N.P. Davies, M. Wilson, L.M. Harris, K. Natarajan, S. Lateef, L. MacPherson, S. Sgouros, R.G. Grundy, T. Arvanitis, A. Peet, Identification and characterization of childhood cerebellar tumors by in vivo proton MRS. NMR Biomed. 21, 908–918 (2008)
L.M. Harris, N. Davies, L. Macpherson, K. Foster, S. Lateef, K. Natarajan, S. Sgouros, M.A. Brundler, T. Arvanitis, R. Grundy, A. Peet, The use of short-echo-time 1H MRS for childhood cerebellar tumors prior to histopathological diagnosis. Pediatr. Radiol. 37, 1101–1109 (2007)
J. Vicente, E. Fuster-Garcia, S. Tortajada, J.M. García-Gómez, N. Davies, K. Natarajan, M. Wilson, R.G. Grundy, P. Wesseling, D. Monleón, B. Celda, M. Robles, A.C. Peet, Accurate classification of childhood brain tumours by in vivo 1H MRS—a multi-centre study. Eur. J. Cancer 49, 658–667 (2013)
S.J. Hipp, E. Steffen-Smith, D. Hammoud, J.H. Shih, R. Bent, K.E. Warren, Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro Oncol. 13, 904–909 (2011)
K.J. Marcus, L.G. Astrakas, D. Zurakowski, M.K. Zarifi, D. Mintzopoulos, T.Y. Poussaint, D.C. Anthony, U. De Girolami, P. Black, N.J. Tarbell, A.A. Tzika, Predicting survival of children with CNS tumors using proton MRSI biomarkers. Int. J. Oncol. 30, 651–657 (2007)
M. Wilson, C.L. Cummins, L. MacPherson, Y. Sun, K. Natarajan, R.G. Grundy, T.N. Arvanitis, R.A. Kauppinen, A.C. Peet, Magnetic resonance spectroscopy metabolite profiles predict survival in pediatric brain tumours. Eur. J. Cancer 49, 457–464 (2013)
S. Blamek, D. Larysz, K. Ficek, M. Sokoł, L. Miszczyk, R. Tarnawski, MRS evaluation of brain tissue damage after treatment for pediatric brain tumors. Acta Neurochirurg. (Suppl.) 106, 183–186 (2010)
S. Rueckriegel, P.H. Driever, H. Bruhn, Supratentorial neurometabolic alterations in pediatric survivors of posterior fossa tumors. Int. J. Radiat. Oncol. Biol. Phys. 82, 1135–1141 (2012)
A.A. Tzika, L.G. Astrakas, M.K. Zarifi, N. Petridou, T.Y. Poussaint, L. Goumnerova, D. Zurakowski, D.C. Anthony, P.M. Black, Multi-parametric MR assessment of pediatric brain tumors. Neuroradiology 45, 1–10 (2003)
A.A. Tzika, D. Zurakowski, T.Y. Poussaint, L. Goumnerova, L.G. Astrakas, P.D. Barnes, D.C. Anthony, A.L. Billett, N.J. Tarbell, R.M. Scott, P.M. Black, Proton magnetic spectroscopic imaging of the child’s brain: the response of tumors to treatment. Neuroradiology 43, 169–177 (2001)
M. Wilson, N.P. Davies, M.A. Brundler, C. McConville, R.G. Grundy, A.C. Peet, High resolution magic angle spinning 1H NMR of childhood brain and nervous system tumors. Mol. Cancer 8, 11 (2009)
L.M. Harris, N.P. Davies, L. Macpherson, S. Lateef, K. Natarajan, M.A. Brundler, S. Sgouros, M.W. English, T.N. Arvanitis, R.G. Grundy, A.C. Peet, Magnetic resonance spectroscopy in the assessment of pilocytic astrocytomas. Eur. J. Cancer 44, 2640–2647 (2008)
F. Yamasaki, K. Kurisu, Y. Kajiwara, Y. Watanabe, T. Takayasu, Y. Akiyama, T. Saito, R. Hanaya, K. Sugiyama, Magnetic resonance spectroscopic detection of lactate is predictive of a poor prognosis in patients with diffuse intrinsic pontine glioma. Neuro Oncol. 13, 791–801 (2011)
R. Kreis, T. Ernst, B.D. Ross, Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn. Reson. Med. 30, 424–437 (1993)
Dž Belkić, K. Belkić, A meta-analysis of studies using MR spectroscopy for evaluating suspicious lesions after radiation therapy of primary brain tumors. J. Math. Chem. 50, 2527–2557 (2012)
Dž Belkić, Quantum-Mechanical Signal Processing and Spectral Analysis (Institute of Physics Publishing, Bristol, 2005)
Dž Belkić, Strikingly stable convergence of the Fast Padé transform (FPT) for high-resolution parametric and non-parametric signal processing of Lorentzian and non-Lorentzian spectra. Nucl. Instrum. Methods Phys. Res. A 525, 366–371 (2004)
P.A. Bottomley, The trouble with spectroscopy papers. J. Magn. Reson. Imaging 2, 1–8 (2002)
B. Ciskowska-Lyson, L. Krolicki, A. Teska, A. Janowicz-Zebrowska, K. Zajda, M. Krzakowski, E. Tacikowska, Proton magnetic resonance spectroscopy investigations in brain metabolic changes after first doses of chemotherapy. MAGMA 15(Suppl. 1), 149 (2002)
E.R. Danielsen, B. Ross, Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases (Marcel Dekker Inc, New York, 1999)
M. Kaminogo, H. Ishimaru, M. Morikawa, M. Ochi, R. Ushijima, M. Tani, Y. Matsuo, J. Kawakubo, S. Shibata, Diagnostic potential of short echo time MR spectroscopy of gliomas with single-voxel and point-resolved spatially localized proton spectroscopy of brain. Neuroradiology 43, 353–363 (2001)
C.K. Kim, B.K. Park, Update of prostate magnetic resonance imaging at 3T. J. Comput. Assist. Tomogr. 32, 163–172 (2008)
J.D. Rabinov, P.L. Lee, F.G. Barker, D.N. Louis, G.R. Harsh, G.R. Cosgrove, E.A. Chiocca, A.F. Thornton, J.S. Loeffler, J.W. Henson, R.G. Gonzalez, In vivo 3T MR spectroscopy in the distinction of recurrent glioma versus radiation effects: initial experience. Radiology 225, 871–879 (2002)
D. Vigneron, A. Bollen, M. McDermott, L. Wald, M. Day, S. Moyher-Noworolski, R. Henry, S. Chang, M. Berger, W. Dillon, S. Nelson, Three-dimensional magnetic resonance spectroscopic imaging of histologically confirmed brain tumors. Magn. Reson. Imaging 19, 89–101 (2001)
E. Hattingen, U. Pilatus, K. Franz, F.E. Zanella, H. Lanfermann, Evaluation of optimal echo time for 1H spectroscopic imaging of brain tumors at 3 tesla. J. Magn. Reson. Imaging 26, 427–431 (2007)
A.C. Peet, S. Lateef, L. MacPherson, K. Natarajan, S. Sgouros, R.G. Grundy, Short echo time 1H magnetic resonance spectroscopy of childhood brain tumors. Childs Nerv. Syst. 23, 163–169 (2007)
A.A. Tzika, L.L. Cheng, L. Goumnerova, J.R. Madsen, D. Zurakowski, L.G. Astrakas, M.K. Zarifi, R.M. Scott, D.C. Anthony, R.G. Gonzalez, P.M. Black, Biochemical characterization of pediatric brain tumors by using in vivo and ex vivo magnetic resonance spectroscopy. J. Neurosurg. 96, 1023–1031 (2002)
K. Glunde, J. Jiang, S.A. Moestue, I.S. Gribbestad, MRS/MRSI guidance in molecular medicine: targeting choline and glucose metabolism. NMR Biomed. 24, 673–690 (2011)
S.W. Provencher, Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30, 672–679 (1993)
Dž Belkić, Exact quantification of time signals in Padé-based magnetic resonance spectroscopy. Phys. Med. Biol. 51, 2633–2670 (2006)
Dž Belkić, Exponential convergence rate of the FPT for exact quantification in magnetic resonance spectroscopy. Phys. Med. Biol. 51, 6483–6512 (2006)
Dž Belkić, Exact signal-noise separation by Froissart doublets in the fast Padé transform for magnetic resonance spectroscopy. Adv. Quantum Chem. 56, 95–179 (2009)
Dž Belkić, Error analysis through residual frequency spectra in the fast Padé transform (FPT). Nucl. Instrum. Methods Phys. Res A 525, 379–386 (2004)
Dž Belkić, K. Belkić, The fast Padé transform in magnetic resonance spectroscopy for potential improvements in early cancer diagnostics. Phys. Med. Biol. 50, 4385–4408 (2005)
Dž Belkić, K. Belkić, In vivo magnetic resonance spectroscopy by the fast Padé transform. Phys. Med. Biol. 51, 1049–1075 (2006)
Dž Belkić, Machine accurate quantification in magnetic resonance spectroscopy. Nucl. Instrum. Methods Phys. Res. A 580, 1034–1040 (2007)
Dž Belkić, K. Belkić, The potential for practical improvements in cancer diagnostics by mathematically optimized magnetic resonance spectroscopy. J. Math. Chem. 49, 2408–2440 (2011)
Dž Belkić, K. Belkić, Molecular imaging and magnetic resonance for improved target definition in radiation oncology, in Radiation Damage to Biomolecular Systems, ed. by G. Gómez, M.C. Fuss (Springer, Berlin, 2012), pp. 411–429
J. Frahm, H. Bruhn, M.L. Gyngell, K.D. Merboldt, W. Hänicke, R. Sauter, Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn. Reson. Med. 9, 79–93 (1989)
Dž Belkić, K. Belkić, Unequivocal disentangling genuine from spurious information in time signals: clinical relevance in cancer diagnostics through magnetic resonance spectroscopy. J. Math. Chem. 44, 884–912 (2008)
Dž Belkić, K. Belkić, Quantification by the fast Padé transform of magnetic resonance spectroscopic data encoded at 1.5 T. J. Math. Chem. 54, 602–655 (2016)
I. Tkáč, P. Andersen, G. Adriany, H. Merkle, K. Uğurbil, R. Gruetter, In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn. Reson. Med. 46, 451–456 (2001)
Dž Belkić, K. Belkić, Strategic steps for advanced molecular imaging with magnetic resonance-based diagnostic modalities. Technol. Cancer Res. Treat. 14, 119–142 (2015)
Dž Belkić, K. Belkić, Resolution enhancement as a key step towards clinical implementation of Padé-optimized magnetic resonance spectroscopy for diagnostic oncology. J. Math. Chem. 51, 2608–2637 (2013)
N.A. Sibtain, F.A. Howe, D.E. Saunders, The clinical value of proton magnetic resonance spectroscopy in adult brain tumors. Clin. Radiol. 62, 109–119 (2007)
E. Hattingen, P. Raab, K. Franz, F.E. Zanella, H. Lanfermann, U. Pilatus, Myo-inositol: a marker of reactive astrogliosis in glial tumors? NMR Biomed. 21, 233–241 (2008)
C. Majós, C. Aguilera, M. Juliá-Sapé, A. León, Á. Rovira, C. Arús, Proton MR spectroscopy improves discrimination between tumor and pseudotumoral lesion in solid brain masses. Am. J. Neuroradiol. 30, 544–551 (2009)
N. Fayed, E. Gonzalez-Toledo, in Magnetic Resonance Imaging and Spectroscopy, Volume 3 of Comprehensive Biomedical Physics, ed. by Dž. Belkić, K. Belkić (Elsevier, Amsterdam, 2014), pp. 273–285
Acknowledgments
This work was supported by the King Gustav the 5th Jubilee Fund and FoUU through Stockholm County Council to which the authors are grateful.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belkić, D., Belkić, K. Improving the diagnostic yield of magnetic resonance spectroscopy for pediatric brain tumors through mathematical optimization. J Math Chem 54, 1461–1513 (2016). https://doi.org/10.1007/s10910-016-0632-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-016-0632-9